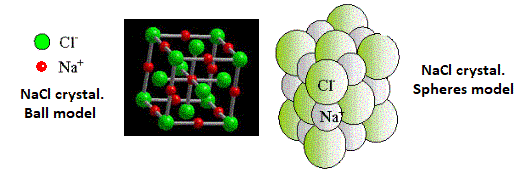
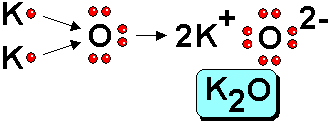When a small amount of sodium is brought into contact with chlorine, a very violent reaction occurs, although not spontaneously. It is necessary to overcome an activation energy. This phenomenon is very common in chemistry. There are reactions where a lot of energy is released that needs some initial "help" for them to occur. But once started, they are so violent that they can cause an accident if you do not act with caution.