An atom is
constituted by:
-
a central nucleus
-
electrons
(negatively charged) that turn around it.
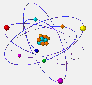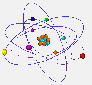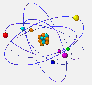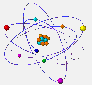
Electrons move around the nucleus
|
|
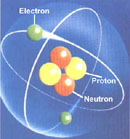
Schematic representation
of the parts of an atom
|
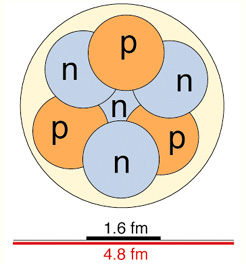
The atomic core is
made up of nucleons that are
of two fundamental classes:
-
protons (positively charged)
-
neutrons (without any charge)
The number of
protons and neutrons of the nucleus defines the various isotopes
of an element.
|
|
|
|
The electrons reside
in atomic orbitals
that have defined shapes
|
Atomic orbitals
of the element sodium
|
Atomic orbitals are distributed in shells (1, 2, 3, etc.) and are designated
letters (s, p, d, f). Each of them has a specific way in which
electrons reside.
S orbitals are spherical. The others have a shape similar to that of two balloons joined at the inflation mouth.
|
|
|
|
|
The size of
the atoms is really tiny. That of its particles is
even smaller so, believe it or not, an atom is a
essentially empty entity.
|
A hydrogen atom consists of only one proton
and one electron. It can be considered as a sphere with a radius of 0.0000001
mm. Its nucleus in turn is another sphere of radius 0.000000000001 mm,
that is, 100,000 times smaller. Electrons are even smaller, so tiny that their size has not yet been accurately measured.
Indirect evidence indicates that its size cannot be greater than 0.000000000000001
mm, that is, 1,000 times smaller than the nucleus.
|
With these measurements it is easy to calculate that 99.99999999999999% of the volume of an atom is
just emptyness.
|