|
ISOTOPE: THE
SAME ELEMENT WITH DIFFERENT NUMBER OF NEUTRONS
|
The atoms of the same element,
which logically have the same number of protons in the
nucleus, but which have different number of neutrons, are called isotopes.
|
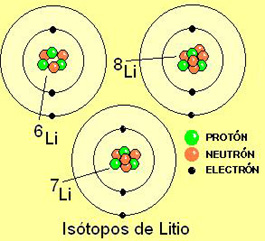
Lithium has
three protons in the nucleus and three electrons on the periphery. But
There are three isotopes of lithium that have three, four and five neutrons
in the core.
|
The isotopes of an element
have the same number of protons in the nucleus and electrons
on the periphery. They only differ in the number of neutrons
and, therefore, in its mass.
|
|
|
|
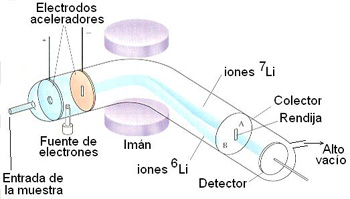
Scheme of a mass spectrometer
|
The isotopes
of an element can be separated and detected in a mass spectrometer. A small amount of the atoms in gaseous state is introduced into a high vacuum tube.
quantity of the atoms to be analyzed in the gaseous state. The atoms
They pass through an area where an electron is knocked out from the atoms by a jet of electrons. In this way the atoms, lacking an electron,
become positively-charged ions. They continue traveling through the tube and reach an area where there is a magnetic field of
a certain intensity. The ions experience here a trajectory
more or less curved, depending on its mass/charge ratio.
Thus, for a certain magnetic field, only the ions
7Li
can reach the slit and are separated from the 6Li ions and viceversa.
|
|
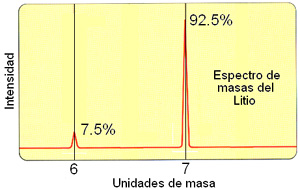
If the
magnetic field is continuously varied and the electrical signal reaching the detector is recorded, we will see that for certain values
of the field a signal with different intensity is detected, which corresponds
to the different isotopes of an atom, of different mass
because they have a different number of neutrons in the nucleus.
So we obtain a mass spectrum of the Lithium atoms
(or any other element), in which the relative intensity of the
signals indicates the different abundance of its isotopes in
Nature.
|
The Li isotope 8 (8Li) has a very low natural abundance and is only detected in ultrasensitive devices.
|
|
