|
|
The electrons are found at the periphery of the atom. They have a very small mass and a tiny size as compared to nucleons. 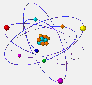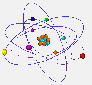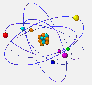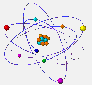 But why
electrons are not attracted to the nucleus and collapse into
it?
|
|
Because the electrons, placed at the periphery of the atom, have restricted zones of movement, called atomic orbitals. |
||
|
S orbitals are spherical. In shell 1 there are only s orbitals. In shell 2 there are s and p orbitals. The latter have a shape similar to the outline of two balloons joined by the filler neck. In the following shells the type of orbitals and its form becomes even more complicated. |
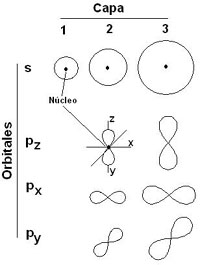
The orbitals here represented are actually superimposed around the nucleus. They are shown separately to increase the clarity of the drawing. |
Atomic orbitals are like
air routes of airplanes that, although invisible, are fixed to avoid collisions. The further away from the nucleus, the more space the orbitals take. |