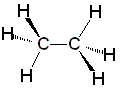
 Ethane
Ethane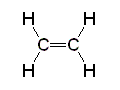 Ethene
Ethene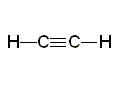 Ethyne
Ethyne| CH3CH3 ethane | CH2CH2 ethene | CHCH ethyne |
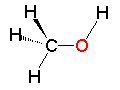 Methanol
Methanol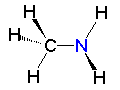 Methylamine
Methylamine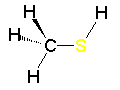 Metiltiol
Metiltiol| CH3OH methanol | CH3NH2 methylamine | CH3SH methylthiol |
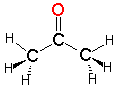 Acetone
Acetone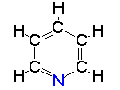 Pyridine
Pyridine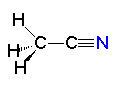 Acetonitrile
Acetonitrile| CH3COCH3 acetone | C5H5N pyridine | CH3CN acetonitrile |