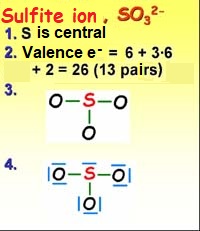
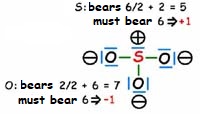Following the four previous rules we can build a more complex structure like that of the sulfite ion.
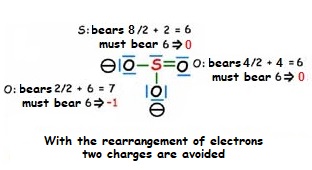
As the molecule has two negative charges, we must account for two more electrons than the 24 provided by the atoms.
1. The central atom is chosen. Which one is less electronegative? Sulfur or oxygen?
2a. The valence electrons (outermost) of all atoms are counted (1 x S = 6, 3 x O = 18, total 24 electrons or 12 pairs)
2b. 2 more electrons are added for the two negative charges: total 26 electrons or 13 pairs)
3. Bonds are formed between the central atom and the peripheral ones.
4. The "excess" electrons are placed on the atoms as unshared pairs (starting with the most electronegative ones).