Nucleophile = keen of nuclei. It refers to an area of a molecule with high electron density. It resembles a base.
Electrophile = keen of electrons. It refers to an area of a molecule with low electron density. It resembles an acid
 The carbon and hydrogens of the methyl group (CH3) are much less electronegative than chlorine.
The carbon and hydrogens of the methyl group (CH3) are much less electronegative than chlorine.
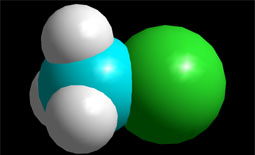
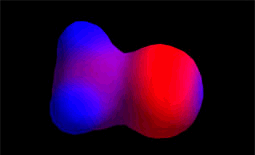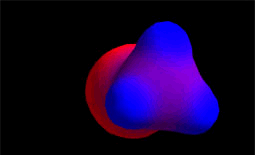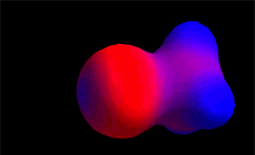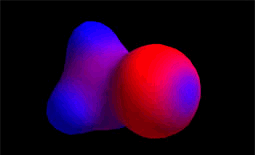 Blue color indicates electron density defect. With which atoms of methyl chloride do the colors match?
Blue color indicates electron density defect. With which atoms of methyl chloride do the colors match?Species "keen of electrons", eager for molecular areas with excess electrons, that is, with relative negative charge density.
Logically, an electrophile will have an electron density defect.
Species "keen of nuclei", eager for molecular areas defective of electrons, that is, with relative positive charge density.
Logically, an nucleophile will have an electron density excess.
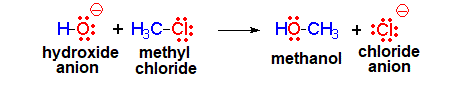 The hydroxide anion (HO-) comes from water due to the loss of a proton. It has a great electronic density. It is the nucleophile (red).
The hydroxide anion (HO-) comes from water due to the loss of a proton. It has a great electronic density. It is the nucleophile (red).