Although atoms join together to form molecules that contain highly stable chemical bonds, these can be broken and/or rearranged.
In this way, different molecules can be formed from other molecules.
If the molecules did not transform into each other, the Universe would not be as we know it and life would not have been possible in it.
Just as it happened to atoms, which formed bonds through their electronic clouds, molecules interact with each other and react by combining or modifying their outermost electrons.
The density of the electronic clouds in a molecule is not uniform and we can find areas where that density is very large and others where it is small.
In general, an area of ??high electron density of a molecule will tend to interact first and then react (share density) with an area of ??low density of another molecule and vice versa.
For a chemical reaction to occur, bonds must be broken and formed between atoms.
Breaking a bond involves an input of energy. On the other hand, the formation of a bond involves the release of energy. If in a reaction more energy is released when forming bonds than is necessary to break them, the process will be energetically favorable. However, this does not mean that the reaction occurs spontaneously.
The oxidation reactions of the biological molecules of our body with the much-needed oxygen in the air are very energetically favorable. Fortunately, they do not occur spontaneously and take place very slowly. Otherwise, life would not be possible.
Therefore, there are two fundamental factors for a chemical reaction to take place:
Favorable energy balance between bonds being broken and formed.
That the reacting molecules meet and collide in the appropriate orientation so that the reaction occurs at a reasonable speed.
Molecules must collide to produce a reaction. If the collision is effective, the reaction occurs.
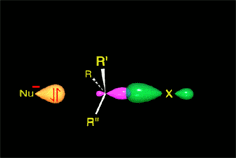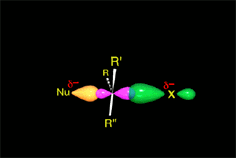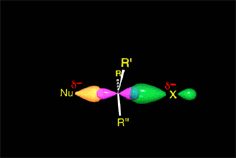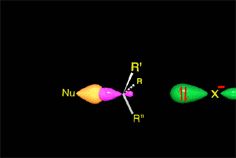
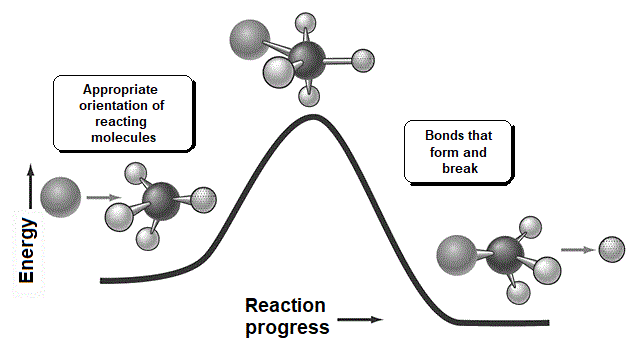 There is a key moment when the bonds that are formed and broken are only half formed or broken (Transition State, TS). Finally you get to the products.
There is a key moment when the bonds that are formed and broken are only half formed or broken (Transition State, TS). Finally you get to the products.
A collision is effective eficaz if the molecules approach each other by orienting their regions of opposite electron density.

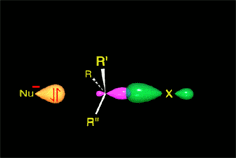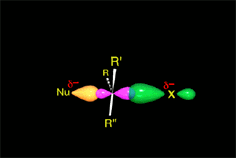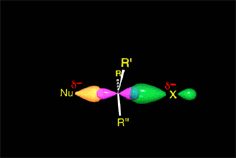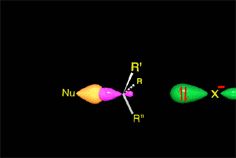
 There is a key moment when the bonds that are formed and broken are only half formed or broken (Transition State, TS). Finally you get to the products.
There is a key moment when the bonds that are formed and broken are only half formed or broken (Transition State, TS). Finally you get to the products.