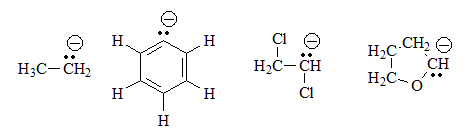
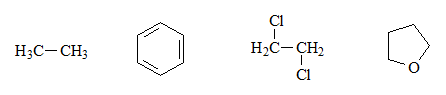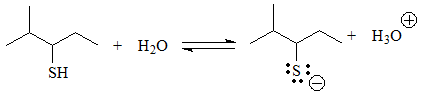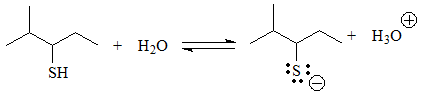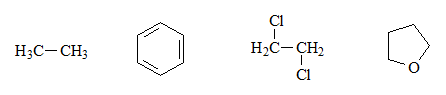Identify the acid groups in the following molecules and write the expected equilibria with water, including the structures of the corresponding conjugate bases:
These four compounds do not have any acid groups per se, because there is no hydrogen bonded to a heteroatom.
Therefore, balance with water does not make sense.
Although equilibrium with water cannot occur, we can imagine the structure of the hypothetical conjugate bases as indicated, by losing a proton. They all have an excess of electrons on a carbon, which is a very high energy situation and, therefore, very unfavorable.
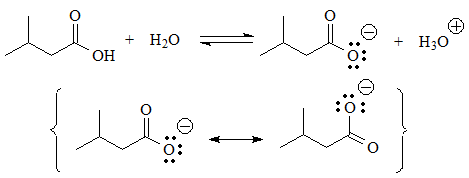
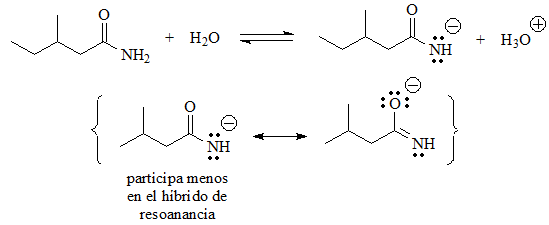
In these cases there is an acid function, because there are hydrogens bonded to heteroatoms.
Equilibrium can be established with water and the conjugate base has a negative charge on a heteroatom that is more electronegative than carbon, which is not a situation as extremely unfavorable as the previous ones. In some cases the charge can be delocalized by resonance, which further stabilizes the conjugate base.
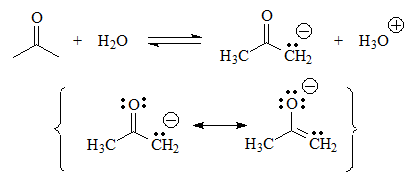
The case of acetone is special. In reality its acidity is very low, but not as much as that of the compounds in the first group studied.
Although there are no hydrogens attached to heteroatoms and the conjugate base has a negative charge on one carbon, acetone shows some acidity because of resonance and can establish equilibrium with water.
The proximity of the C=O group means that the charge can be distributed towards oxygen. Can you write resonant forms in the conjugate base to prove this? Surely yes... There you have it. You try it!
Order the above compounds by groups from highest to lowest acidity.
The most acidic of all will be the one with the COOH group whose nickname is precisely carboxylic "acid".
The conjugate base is the LEAST UNSTABLE of all because the charge is distributed between the two oxygens by resonance.
pKa = 4
The next in acidity could be the amide, very similar in structure to the carboxylic acid, except that instead of an OH group it has an NH2 group.
But the charge is not distributed between identical atoms and therefore the conjugate base is much less stable than the carboxylate.
pKa = 15 (amides are much less acidic than expected)
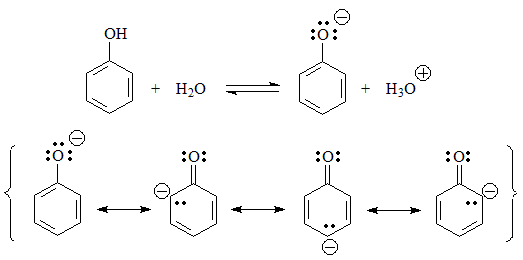
The next group of acids are phenols, in which the negative charge of the conjugate base resides on a heteroatom and can be delocalized by the aromatic ring.
pKa = 10
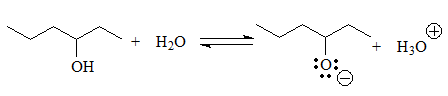
The next group of acids are alcohols and thiols, in which the negative charge of the conjugate base resides on a heteroatom but cannot be delocalized because the O or S are bonded to carbons sp3.
pKa = 13-15
The penultimate acid will be acetone. Although the negative charge in the conjugate base is located on a carbon, which is not very electronegative, next to it is the C=O group with a pi system.
This allows a small delocalization of the electron density that slightly stabilizes the conjugate base.
pKa = 20
The last group of substances to be placed in this order of acidity are those that do not have any hydrogen bonded to a heteroatom.
pKa > 30