In Organic Chemistry - the chemistry of carbon - the so-called 'HETEROATOMS' - atoms different from carbon and hydrogen - are very abundant and they usually bear electron lone pairs.
Consequently, in the organic molecules, there are usually many functions bearing electrons that can be shared with incoming protons and thus show BASICITY properties.
Functional groups bearing unshared electrons can accept protons and behave as more or less powerful bases.
Here you are some examples:
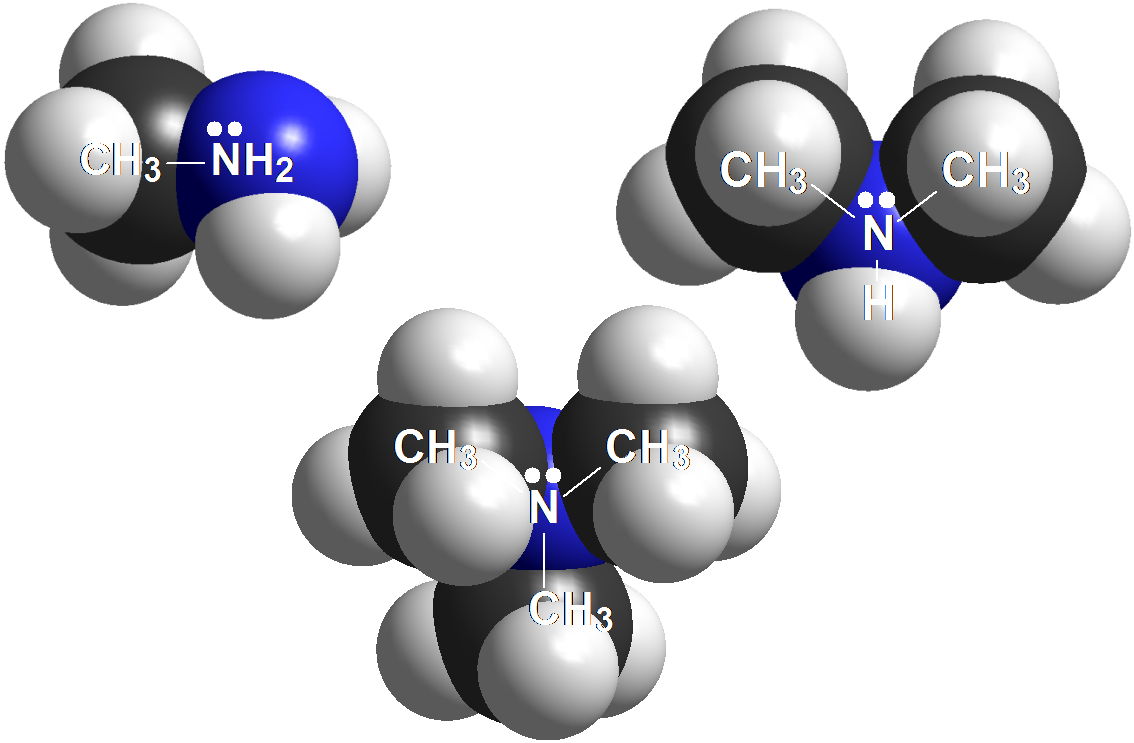 Amines
Amines
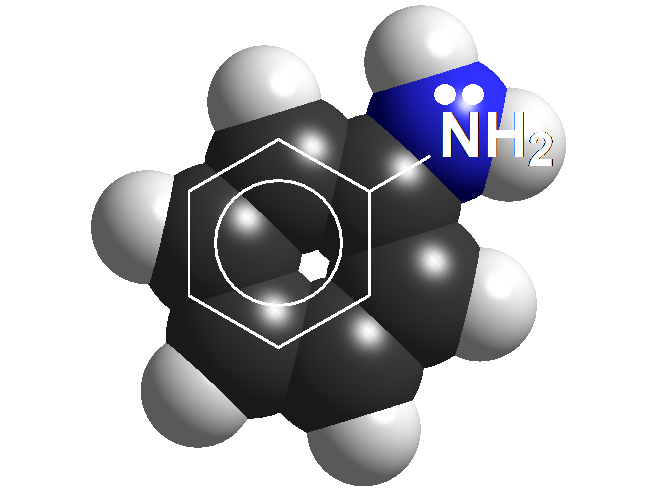 Anilines
Anilines
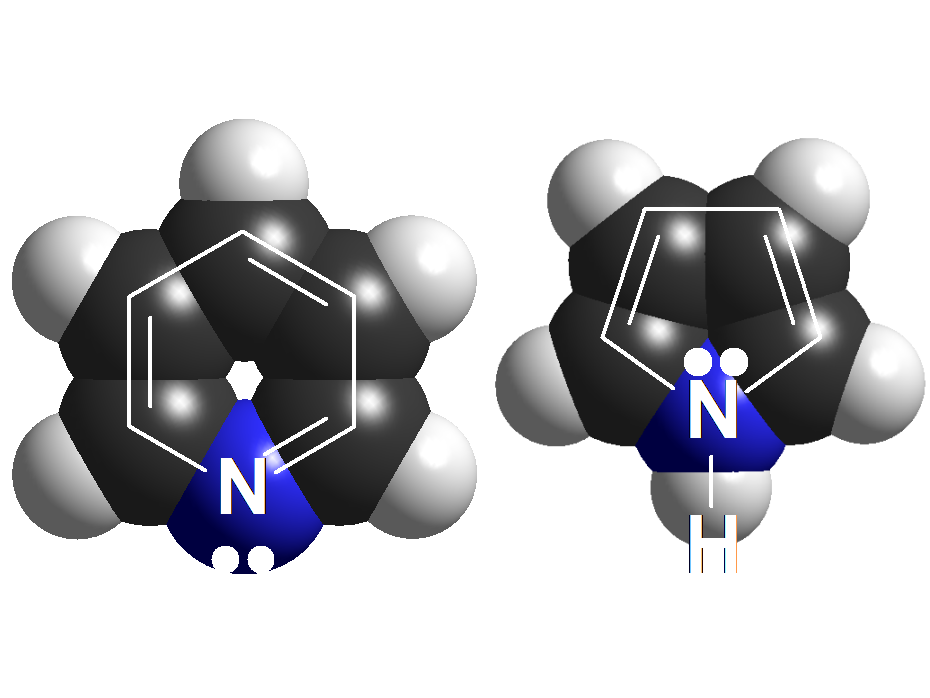 Pyridine vs. Pyrrole
Pyridine vs. Pyrrole
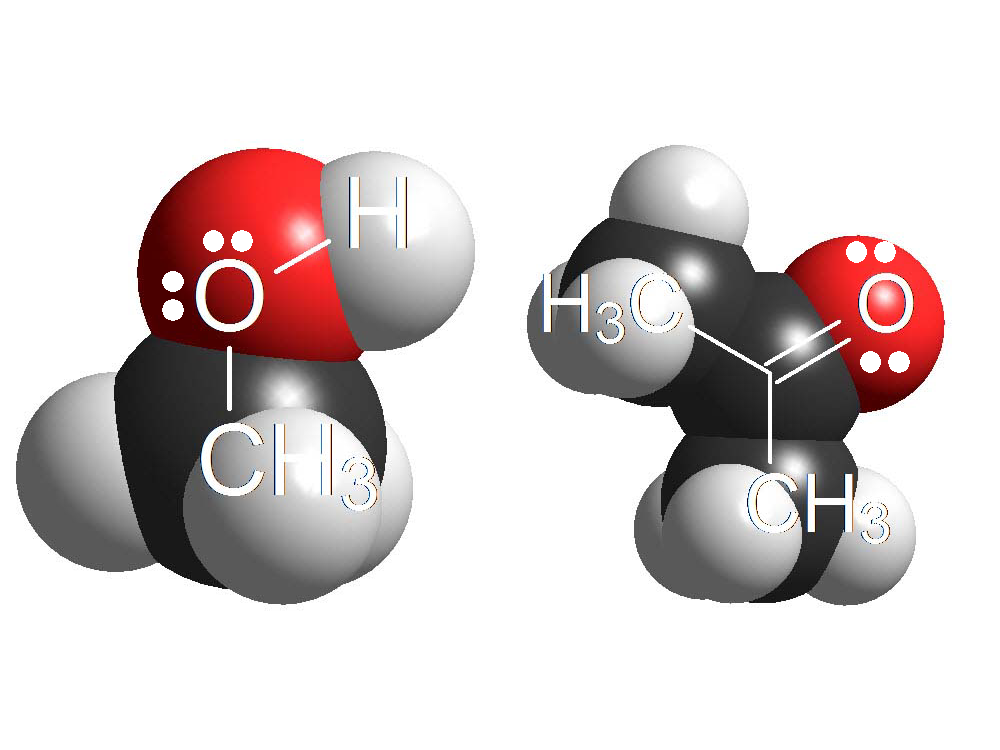 Alcohols and Ketones
Alcohols and Ketones
The assertion 'more or less powerful base' is vague and of low scientific value. Is there any method to measure basicity and give it a number?
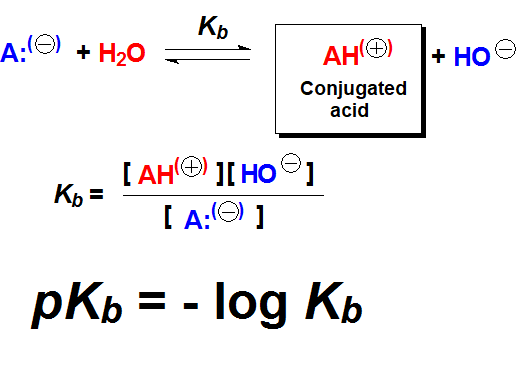
The pKb value is the numeric measurement of basicity and is related to the shift of the acid-base equilibrium of a compound with water.
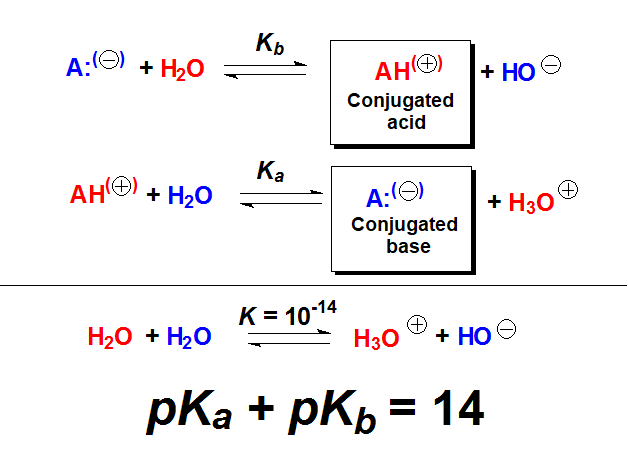
The pKa and pKb values are related by the equilibrium constant of water with itself - water ionic product.
Therefore, from the pKa value of an acid, one easily knows the pKb value of its conjugated base and viceversa.
Which are the pKb values of the aforementioned types of organic compounds? In each kind there is a variable range of pKb values depending on each single structure. We will finely address this problem when studying the particular functional groups but, for the time being, let's give pKb's an introductory touch.
Amines are the strongest bases in Organic Chemistry.
Their pKb are in the range 3-5.
Their conjugated acids, ammonium salts, display an acidity (pKa = 9-11) similar to that of phenols. This is just a coincidence because ammonium salts and phenols have of course nothing to do with one another.
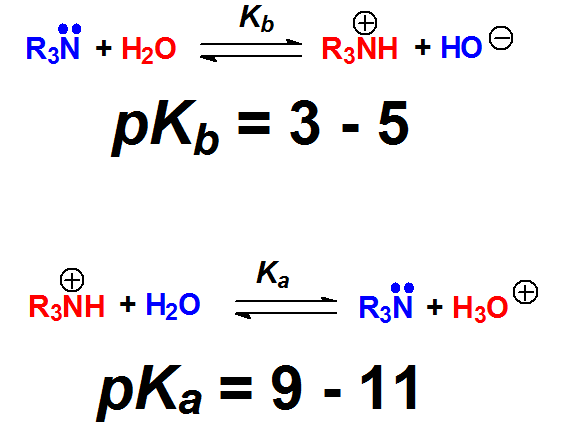 Amines
Amines
 Anilines
Anilines
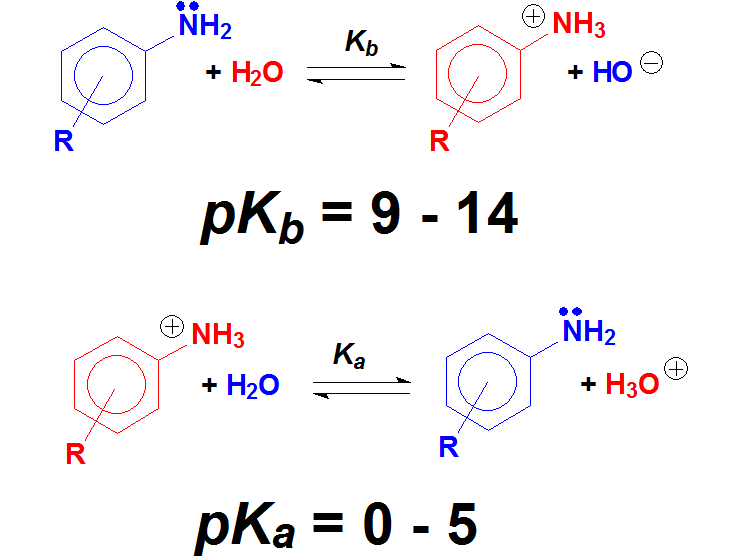
Anilines are structurally very close to amines but notice that the N atom is bonded to an aromatic ring.
And that should make a big difference because anilines are far less basic than amines.
How many times are anilines less basic than amines as an average?
Wrong! It's not 6 times but 10 to 6 times.
Remember that the definition of pKb a logarithm is involved!!!
Please, note that anilines' conjugated bases, anilinium salts, are as acidic as carboxylic acids.
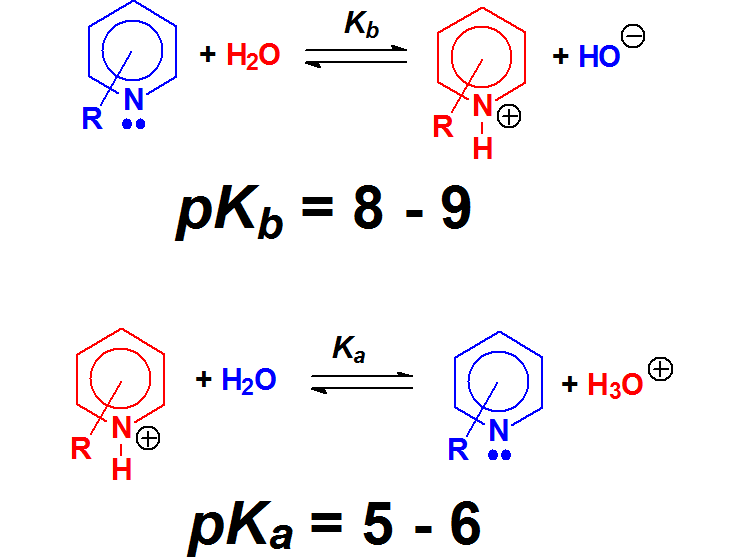
Pyridines display a similar basicity to that of anilines.
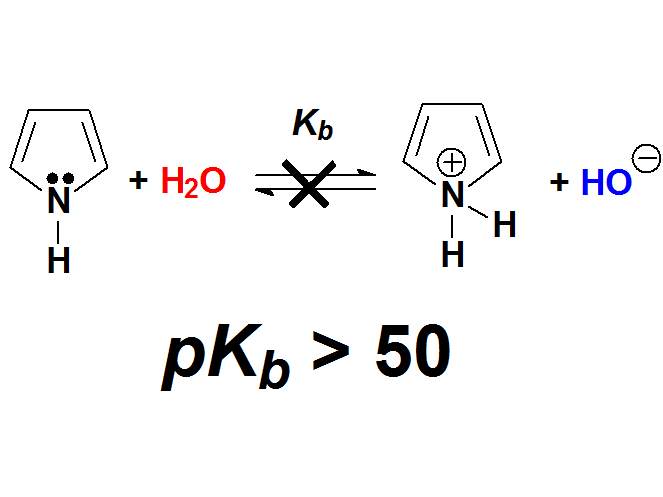
In contrast, Pyrroles, apparently very structurally related to pyridines, display a extremely low basicity.
How's that?
We'll resolve the mistery in full when we study 'AROMATICITY'.
But in a simpler way, pyridine's N atom bears a true unshared pair of electrons residing in a sp2 orbital, whereas pyrrole's N atom has the 'apparent' unshared electron pair in a p orbital that heavily overlaps with the flanking double bonds.
In pyrrole's N atom, the electron lone pair is way, way less available than in pyridine.
 Pyridines vs. Pyrroles
Pyridines vs. Pyrroles
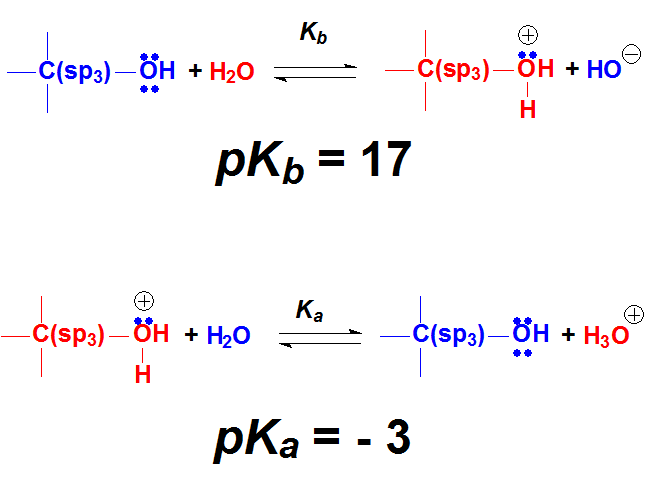
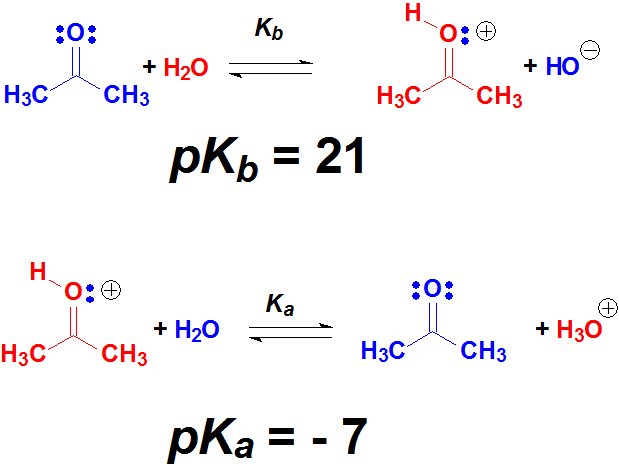 Alcohols and Ketones
Alcohols and Ketones
The oxygen atoms bear two electron lone pairs and the protons of a strong enough acid can bond to them.
When OH or C=O groups 'protonate', the resulting new molecules are pretty reactive.
The beginning of numerous reactions of alcohols and ketones is their 'protonation' with a strong acid.
We'll see that in detail when we study these functional groups in depth.
The stronger the basicity of a compound, the more shifted its equilibrium to the right hand side.
This fact suggests that the stronger the basicity, the more accesible the electron lone pair of the base.
The more accesible the electron lone pair of the base, the more shifted the equilibrium to the right hand side.
In Amines, the electron lone pair
is completely localized at the N atom with moderate electronegativity.
That's an explanation to their basicity strength, usually the highest in Organic Chemistry.
They get 100% with mineral acids.
Anilines experimentaly display a lower basicity than amines, suggesting that the electron lone pair at the N atom is less accesible in anilines than in amines.
We can easily understand that with resonance forms where the electron lone pair is delocalized towards the aromatic ring.
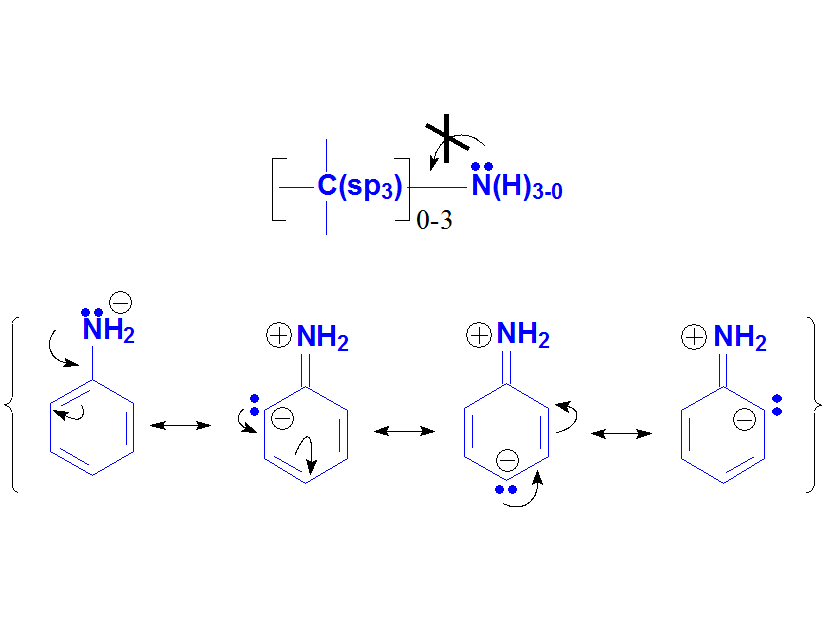 Amines vs. Anilines
Amines vs. Anilines
The N atom of pyridines CANNOT
delocalize its electron lone pair because the possible resonance form produce kind of a linear allenic structure that is impossible to attain within a six-membered ring.
Therefore the electron lone pair is available to an incoming proton and pyridines are basic compounds.
On the contrary, in pyrroles the electron lone pair can be delocalized towards the ring and its availability to an acid is extremely low.
Both pyridines and pyrroles are 'aromatic' - synonimous to additional stability - but the big difference between them is that pyrroles DO need the electron lone pair to attain it whereas pyridines DO NOT.
That will be fully explained in the AROMATICITY chapter.
For the time being, please note that resonance forms are able to give us a reasonable explanation as to the quite different basicity properties of pyridines and pyrroles.
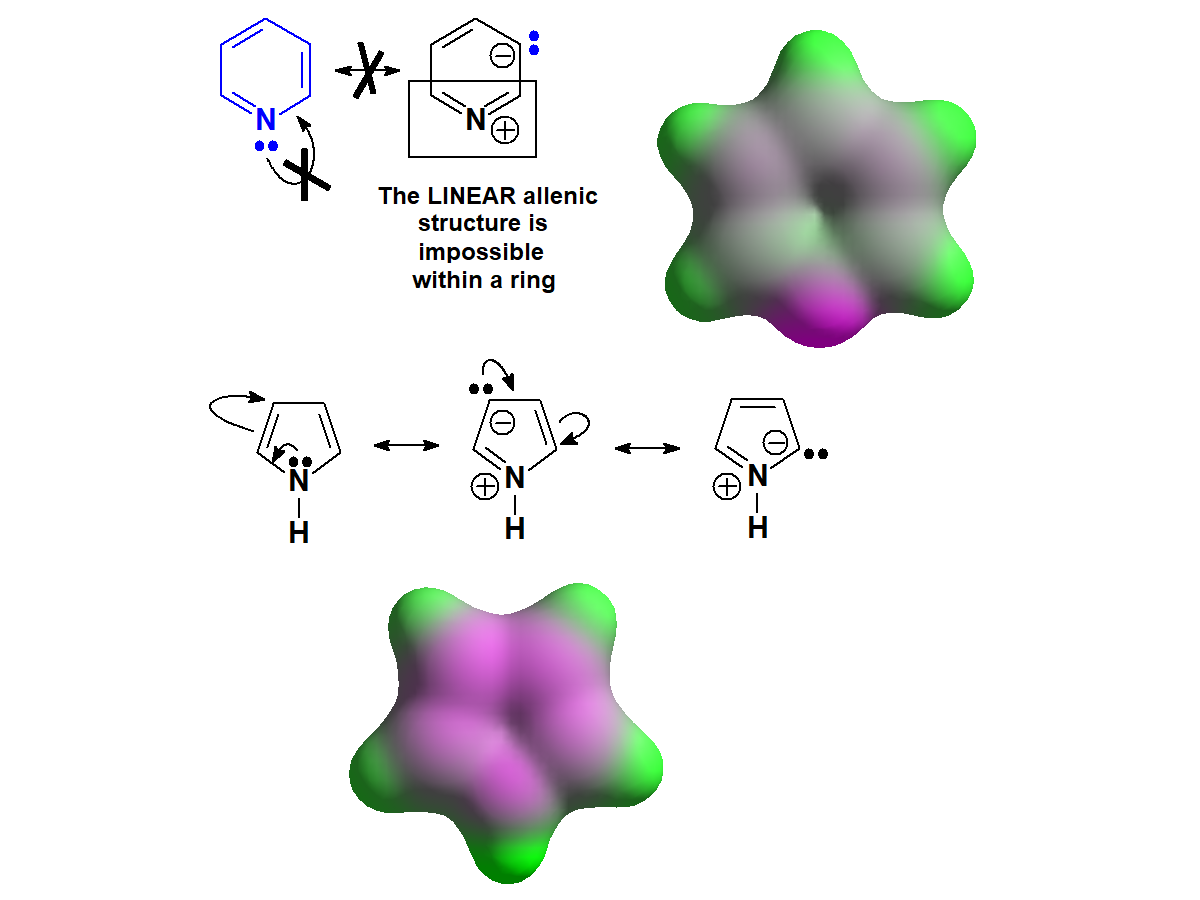 Pyridine vs. Pyrrole
Pyridine vs. Pyrrole
The electron lone pairs on O atoms are weakly basic because they are over a strongly electronegative atom.
But given a sufficiently strong acid, O atoms can 'protonate' in a certain number of molecules.
The resulting few protonated ones are very reactive and can give rise to new products. The equilibrium will regenerate more protonated molecules that would react and so on and so forth.
In many occasions, the first step in the reaction of alcohols or carbonyl (C=O) derivatives is their protonation.
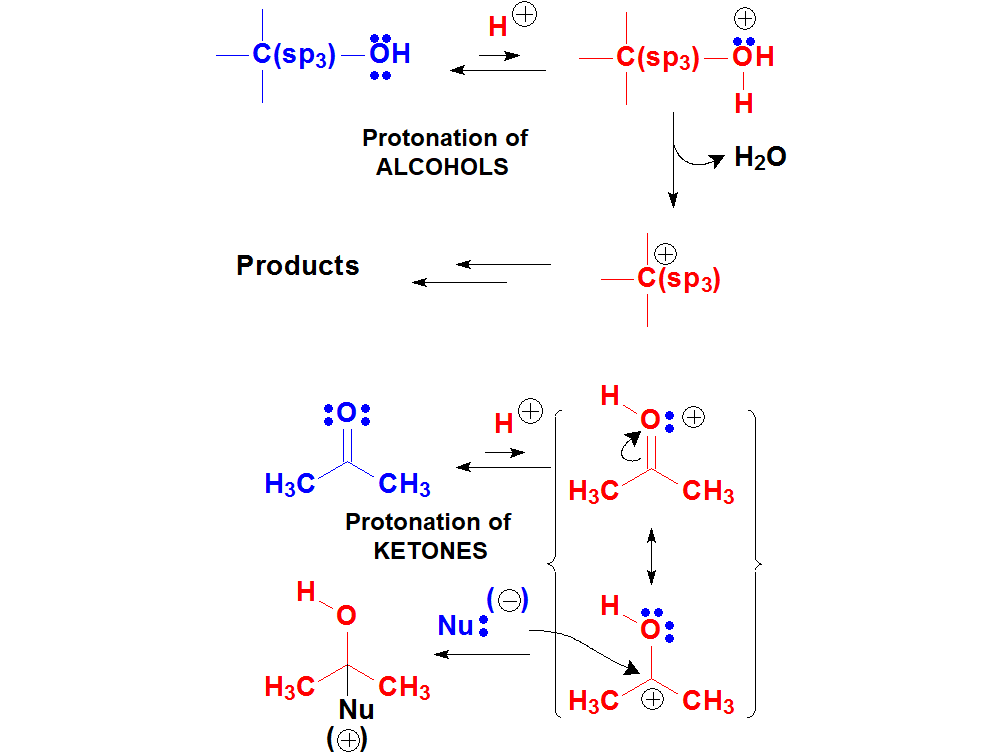 Alcohols vs. Ketones
Alcohols vs. Ketones
 Amines
Amines
 Anilines
Anilines
 Pyridine vs. Pyrrole
Pyridine vs. Pyrrole
 Alcohols and Ketones
Alcohols and Ketones
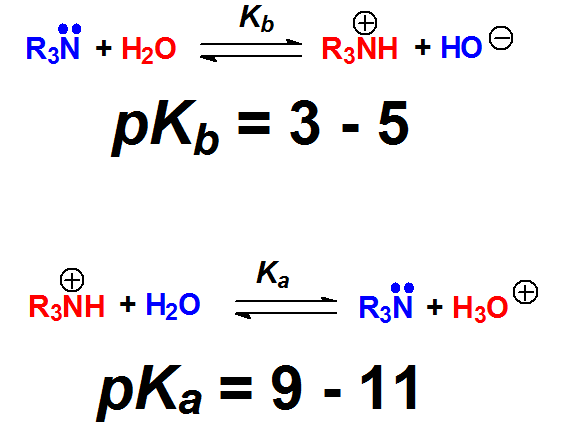 Amines
Amines
 Anilines
Anilines

 Pyridines vs. Pyrroles
Pyridines vs. Pyrroles

 Alcohols and Ketones
Alcohols and Ketones Amines vs. Anilines
Amines vs. Anilines
 Pyridine vs. Pyrrole
Pyridine vs. Pyrrole
 Alcohols vs. Ketones
Alcohols vs. Ketones