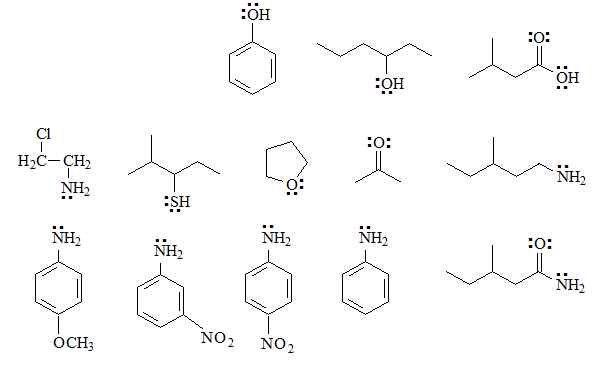
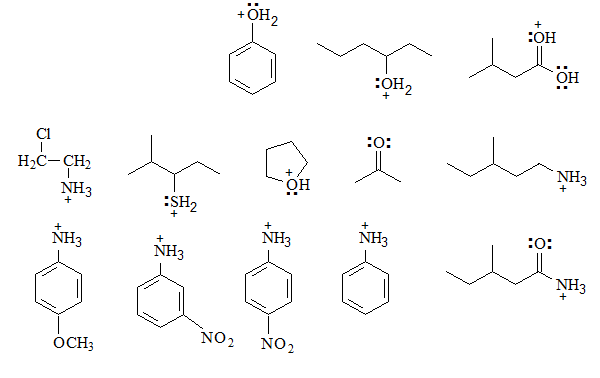
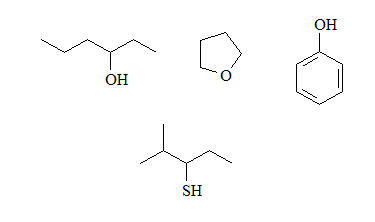
Identify the basic groups in the following molecules and write the expected equilibria with water, including the structures of the corresponding conjugated acids:
These two compounds do not have any basic group per se, because there is no atom with free pairs of electrons that can be given up.
Therefore, balance with water does not make any sense
In these cases there is at least one basic function, because there are heteroatoms with unshared electrons.
Equilibrium can be established with water and the conjugated acid has a positive charge on a heteroatom that is more electronegative than carbon, which is not an extremely favorable situation. Therefore the balance is very shifted towards the base.
Despite this, we can study the relative basicity from the resonant forms of the base, if it is possible to write one.
In the positively charged conjugated acid, resonant forms cannot be written because the heteroatom would be surrounded by 10 electrons.
The more delocalized the electrons are in the starting base by resonance, the less available they will be towards water (which acts as an acid) and the lower their basic strength will be.
Order the above compounds by groups from highest to lowest basicity.
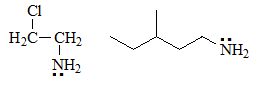
The most basic will be the aliphatic amines in which the unshared electron pair of the nitrogen cannot be delocalized by resonance as the nitrogens are bonded to sp3 carbons.
pKb = 3-5
It is expected that 2-chloroethylamine is a little less basic due to the inductive attracting effect of Cl (electronegativity) that removes electron density from nitrogen.
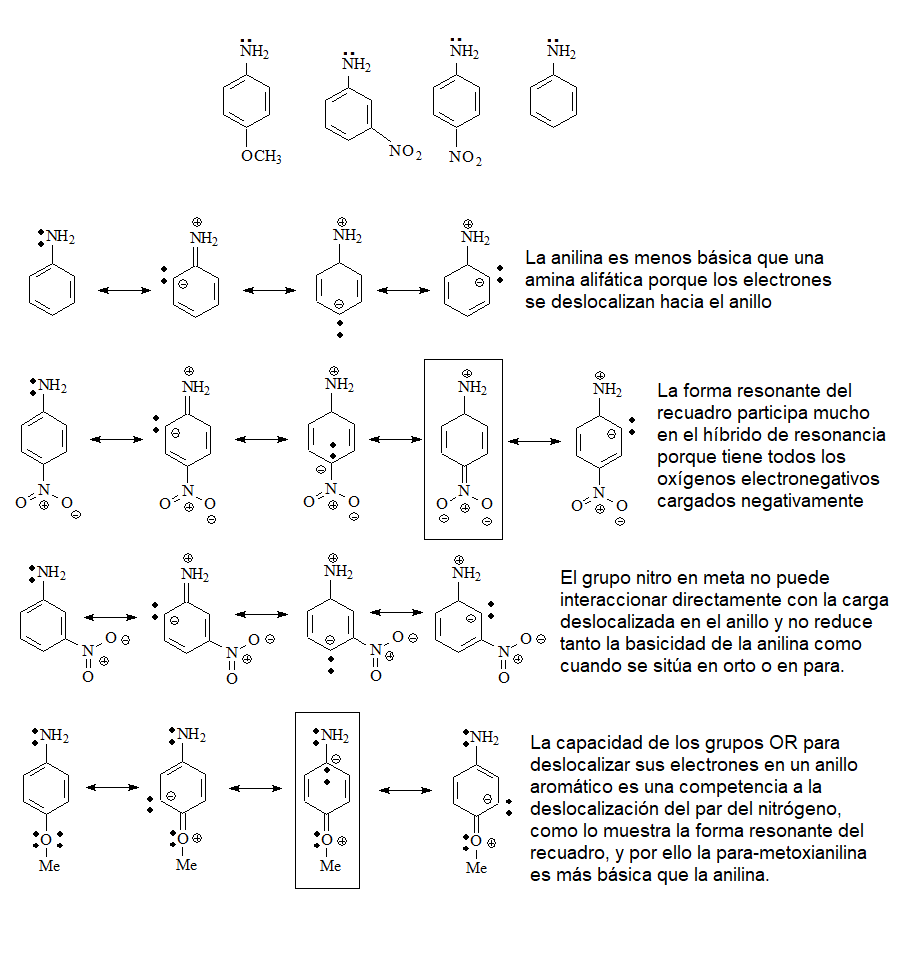
The next in basicity will be the ANILINES
(pKb = 9-14)
in which the electron pair of the nitrogen is less available than in the previous ones due to the effect of resonance with the ring.
Electron withdrawing groups in ortho or para (-M mesomer effect) further reduce basicity.
This is the case of para-nitroaniline. Electron-donating groups (+M mesomer effect), such as the OMe group, increase basicity because they decrease the delocalization of the nitrogen electron pair.
Alcohols and ethers have a small basicity because the oxygen atom is very electronegative and gives up its electrons less easily than a nitrogen.
Phenols will be less basic than alcohols because the electronic density of oxygen can be delocalized towards the ring.
Thiols will be more basic than alcohols because sulfur is less electronegative than oxygen.
pKb = 15-17
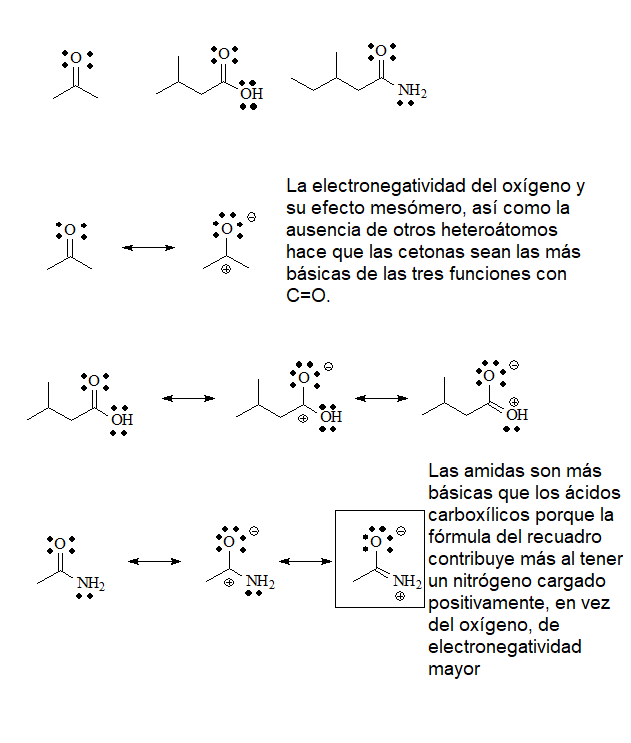
The last group of bases will be those that have the group C=O. In it, the unshared electrons of oxygen reside in sp2 orbitals, with a greater s character and closer to the nucleus, so they will be less available towards an exterior acid. In any case, the protonation of a C=O group by a sufficiently strong acid is very important to increase its reactivity.
pKb = 21