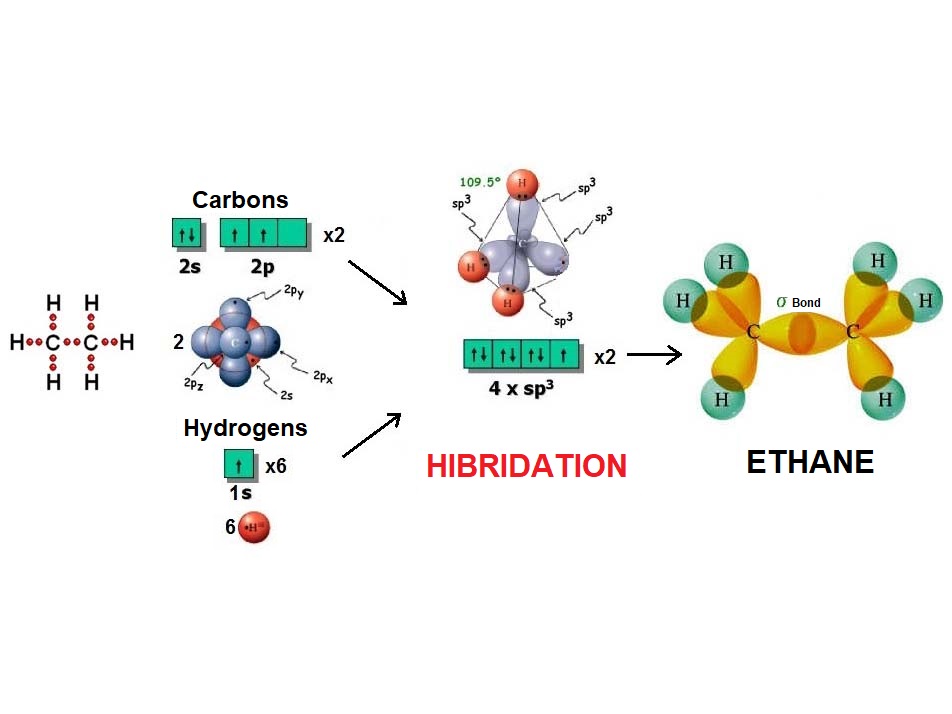
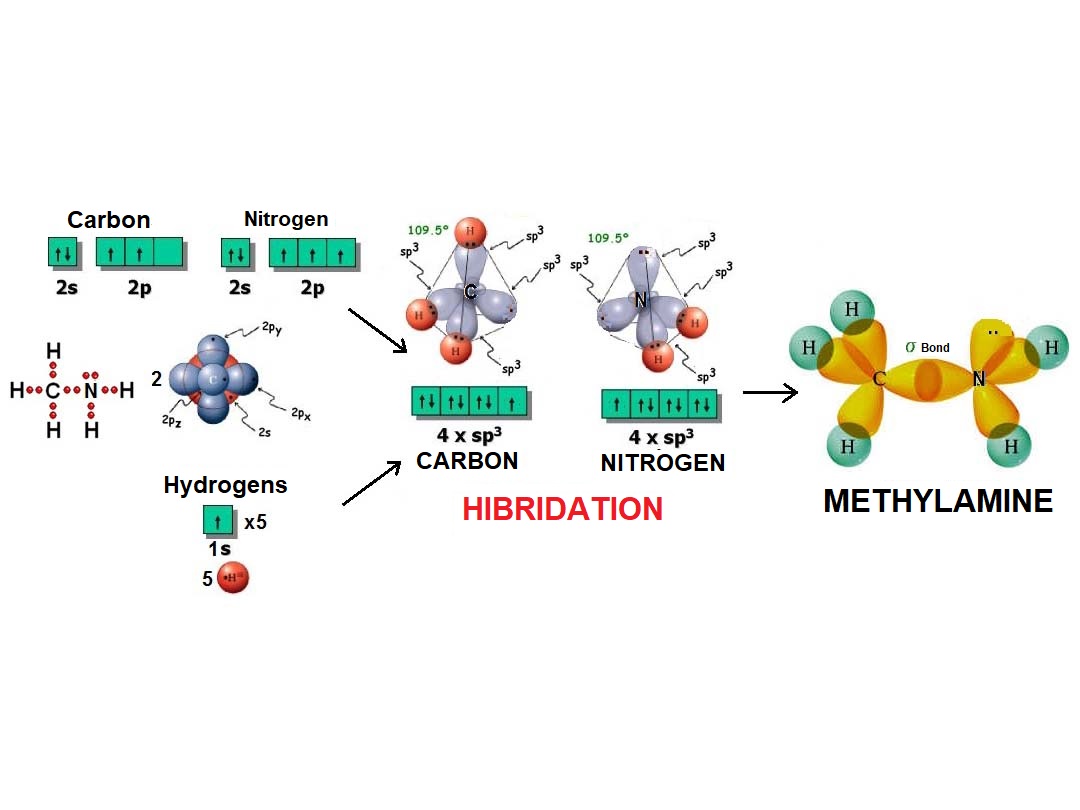
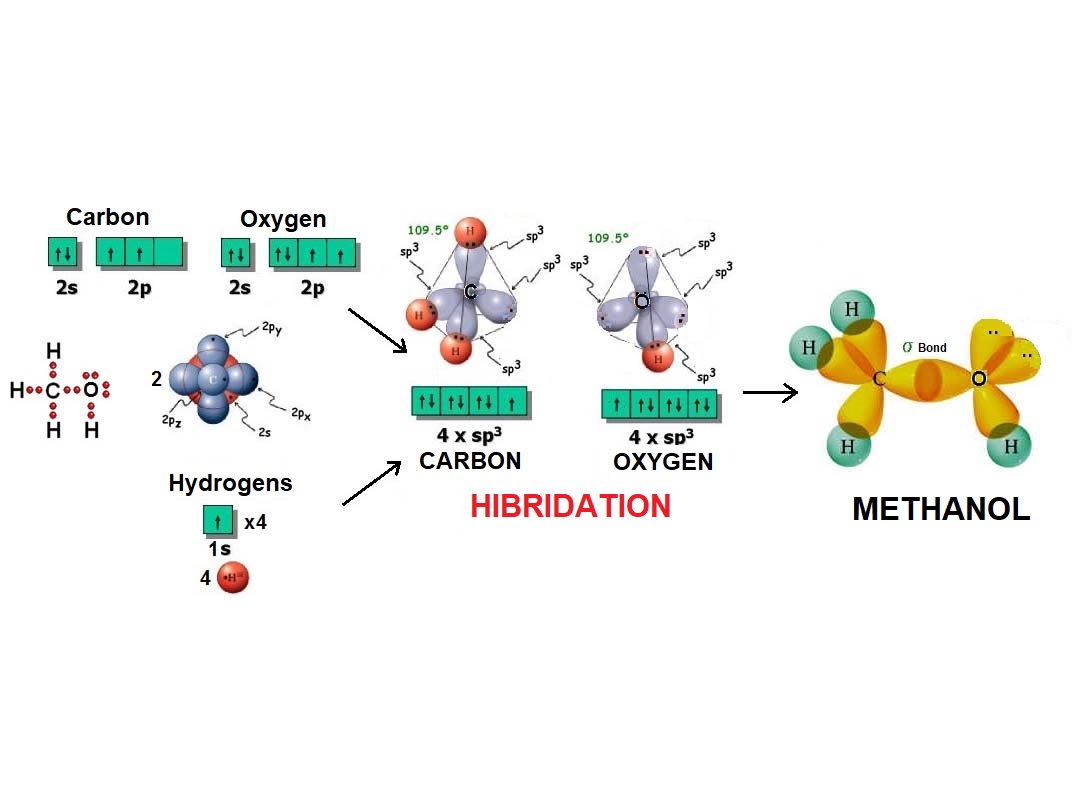
In the same fashion as four balloons arrange towards the vertices of a tetrahedron, four electron densities adopt such arrangement in order to be the farthest possible apart from each other and thus minimize their repulsion

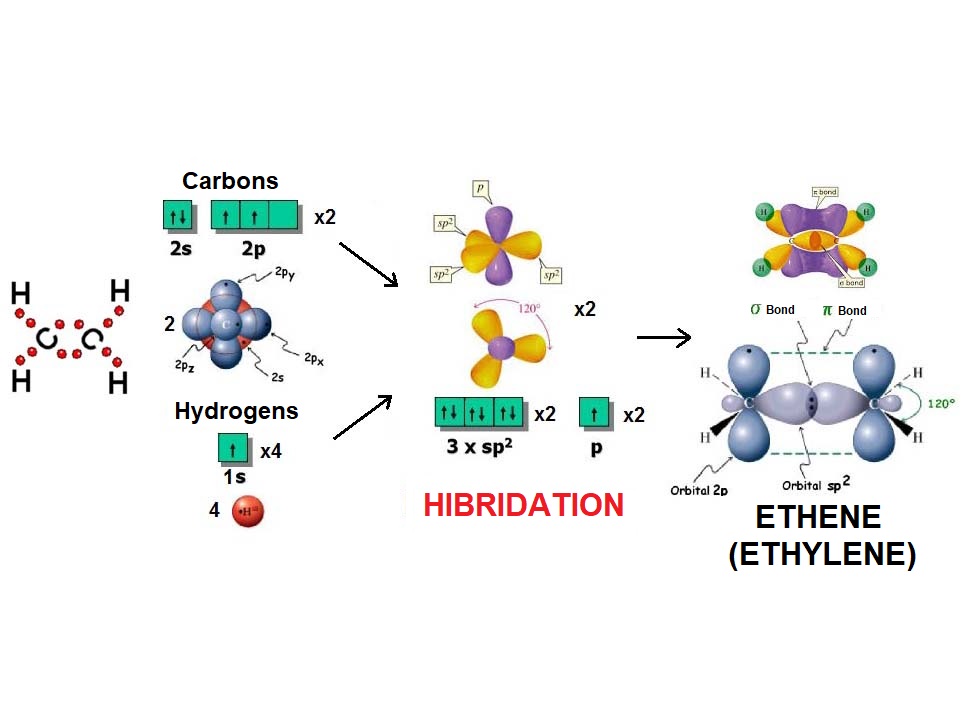
In the same fashion as three balloons arrange towards the vertices of a triangle, three electron densities adopt such arrangement in order to be the farthest possible apart from each other and thus minimize their repulsion.

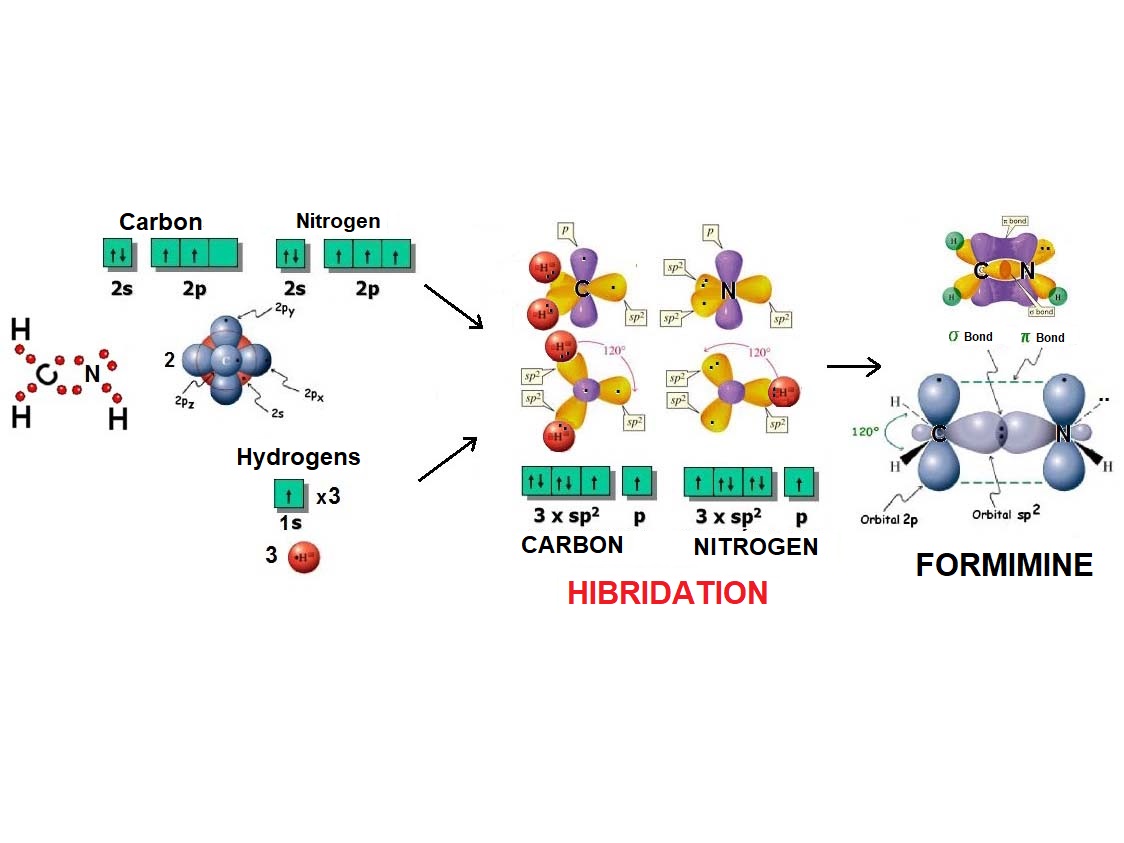

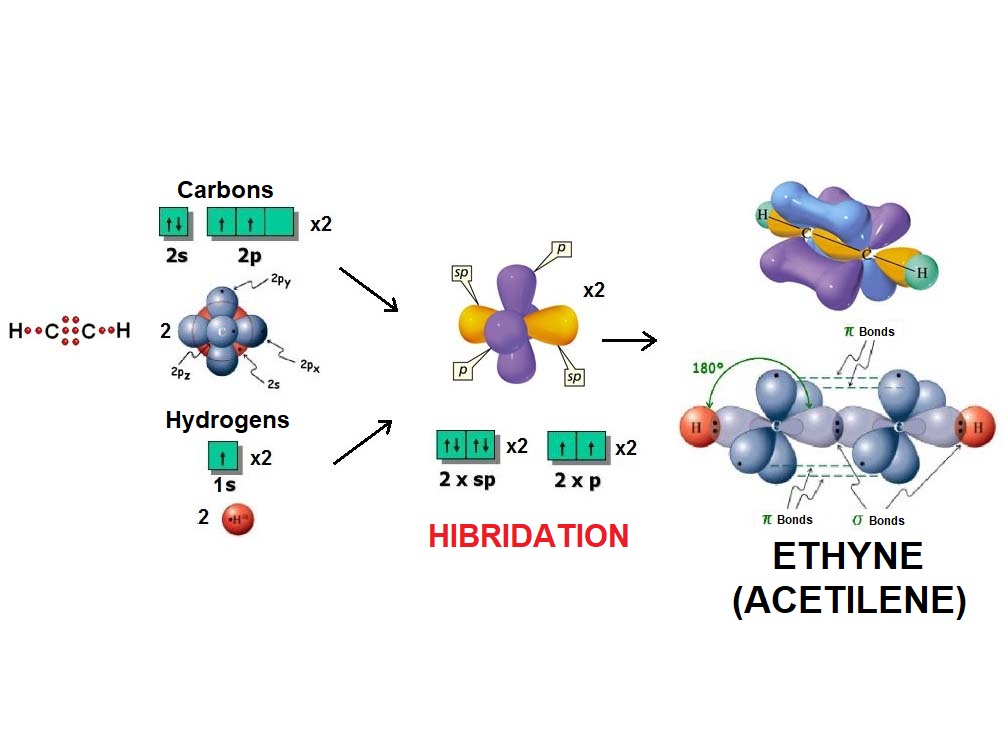
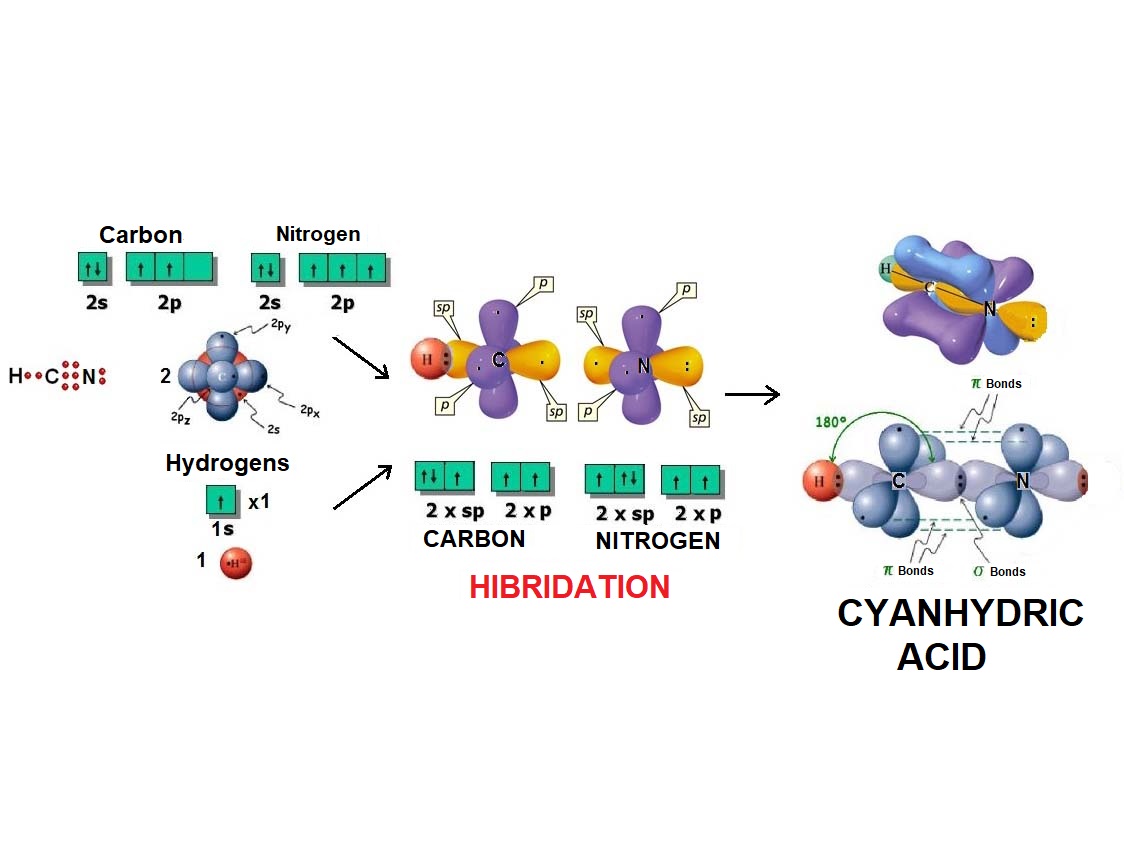
In the same fashion as two balloons arrange towards forming a line, two electron densities adopt such arrangement in order to be the farthest possible apart from each other and thus minimize their repulsion.