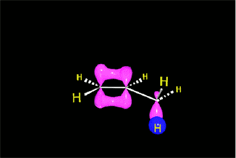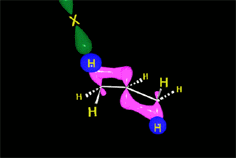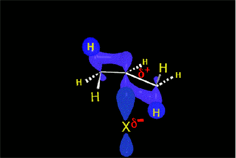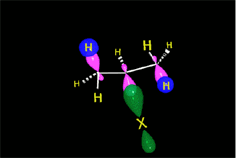
One may understand the reactions between molecules as a function of their electron distribution. The electron density is to the molecules as the atmospheric pressure is to the earth. The latter can be understood by low (storm) and high (anticyclone) pressure zones. Electronegative atoms concentrate electron density and leave their surroundings with a lack of it.
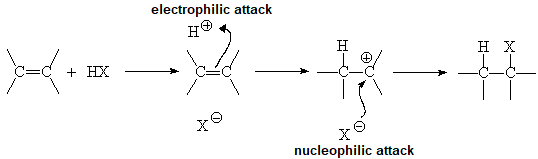
The relatively high electron density at the double bond is electrophilically attacked by the proton of HCl.
A carbocation is thus produced that electrophilically attacks the chloride anion.
The same can be said in a complementary manner:
The relatively high electron density at the double bond nucleophilically attacks the proton of HCl. A carbocation is thus produced that is nucleophilically attacked by the chloride anion.
The two ways of discribing the reaction are correct.
Can you realize the subtlety of the language using the electrophile-nucleophile pair of terms?
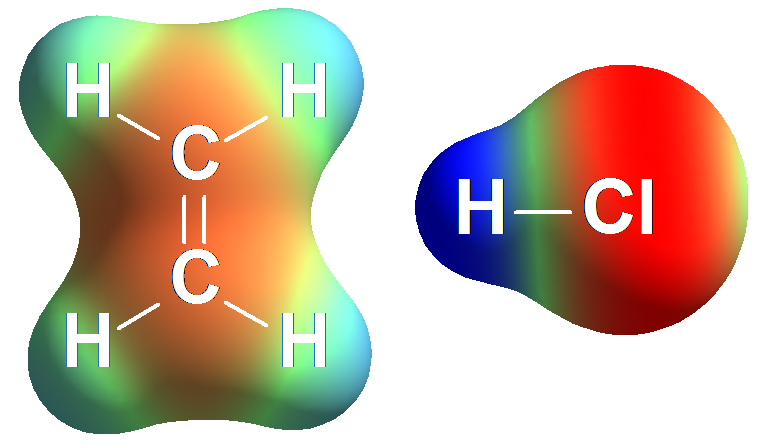 Electron density distribution in ethene (red = high; blue = low)
Electron density distribution in ethene (red = high; blue = low)