STEREOCHEMISTRY OF COMPLEX MOLECULES
An excellent example is that of the sugars (carbohydrates), for instance those with six carbon atoms C6H12O6 (HOCH2-CHOH-CHOH-CHOH-CHOH-CHO) denoted as ALDOHEXOSES.
They bear 4 stereocenters and because of that they have 16 stereoisomers (2 to 4).
Here you are 8 of them. What isomers are still missing?
Emile Hermann Fischer (1852-1919) developed a simple way to represent the stereocenters of such molecules.
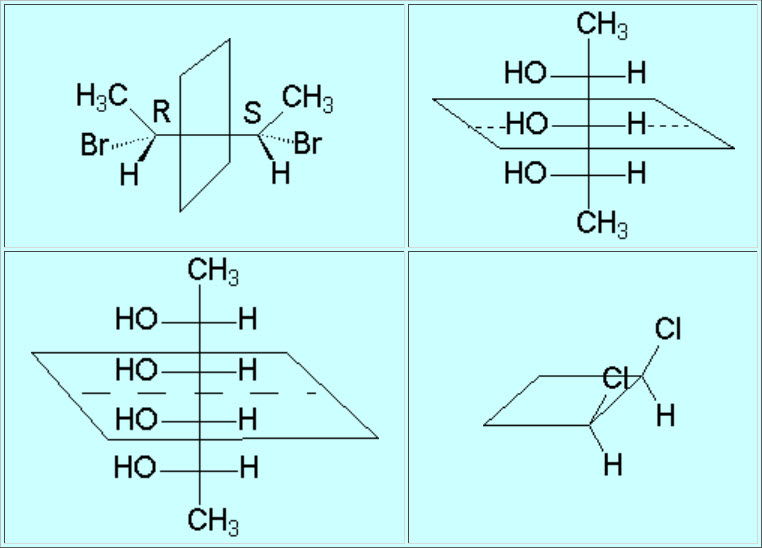
In the so-called Fischer's projections every carbon is drawn as a cross in which the horizontal lines are pointing to the front and the vertical ones to the back.
The stereoisomers that relate each other as object and non-superimposable mirror image are called ENANTIOMERS.
The stereoisomers that DO NOT relate each other as object and non-superimposable mirror image are called DIASTEREOMERS.
ENANTIOMERS go always in pairs. For each enantiomer, every other stereoisomer not being its pair is a diastereomer.
Two equaly substituted stereocenters give rise to only three stereoisomers. One of them is called 'meso' and is not chiral.
These are examples of 'meso' forms.
The 'meso' forms look like chiral because they have various stereocentes.
But the global symmetry of the molecule cancels chirality.
The mirror image of a 'meso' form is always superimposable.