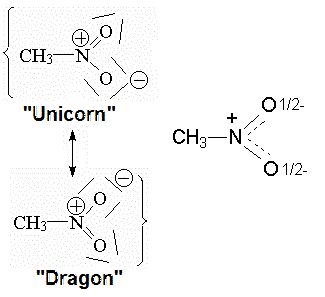
How would you explain to someone who has never seen one what a rhino looks like? You would use similes. For example, you could explain that a real rhinoceros is something, say, intermediate, between a unicorn and a dragon. The explanation may be more or less fortunate, but if the person you talk to knows what unicorns and dragons are, they will have a fairly reasonable idea of what a rhinoceros really is.
The reality of the nitromethane ("rhino") molecule is that the two N-O bonds are completely equivalent in all their properties: strength, N-O distance, etc.
However, the Lewis formula suggests that one bond is N-O and the other is N=O. We need to explain reality with appropriate "unicorns" and "dragons."
Real nitromethane molecule with the two equivalent N-O linkages 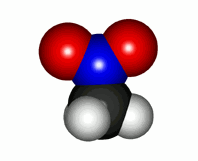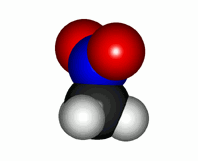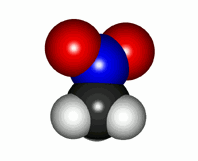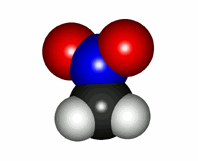
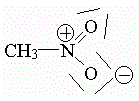 With this Lewis structure alone the reality of nitromethane cannot be explained
With this Lewis structure alone the reality of nitromethane cannot be explained
In this case the "unicorn" and the "dragon" are almost the same. Nitromethane must be described with two very similar Lewis forms, in which only which of the two oxygens supports the double bond with the nitrogen varies. In many cases an "intermediate" formula can be drawn to describe the "resonance" of nitromethane between the two Lewis formulas.
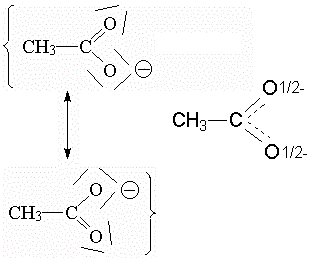
Something similar happens in the acetate ion. The two C-O bonds are in reality equivalent. But in the Lewis formulas one is C-O and the other C=O. The acetate ion "resonates" between the two Lewis formulas. In this case you can also draw an intermediate formula.
1. Resonant structures only involve movement of electrons
(NEVER atoms) from adjacent positions.
2. Resonant structures in which all 2nd period atoms have complete octets are more important (they contribute more to the resonance hybrid) than structures that have incomplete octets.
3. The most important structures are those that involve the minimum charge separation.
4. In cases where a Lewis structure with complete octets cannot be represented without charge separation, the most important structure will be that in which the negative charge is placed on the most electronegative atom and the positive charge on the most electropositive one.
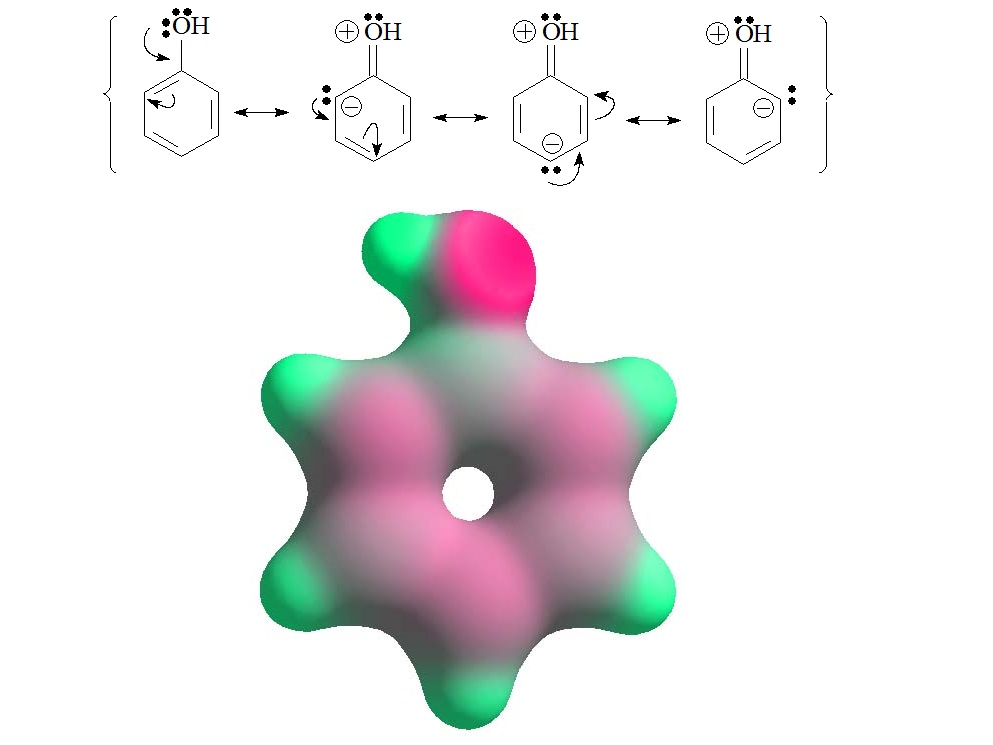
The reality of phenol is that the aromatic ring has a slight excess charge in the "ortho" and "para" positions to the OH group. Four Lewis formulas are needed to explain it.
The predominant form is the first, which has no charge separation. The others, although they place the positive charge on the most electronegative atom, are the only ones that explain why the ring has a slight excess of electronic density in the "ortho" and "para" positions.
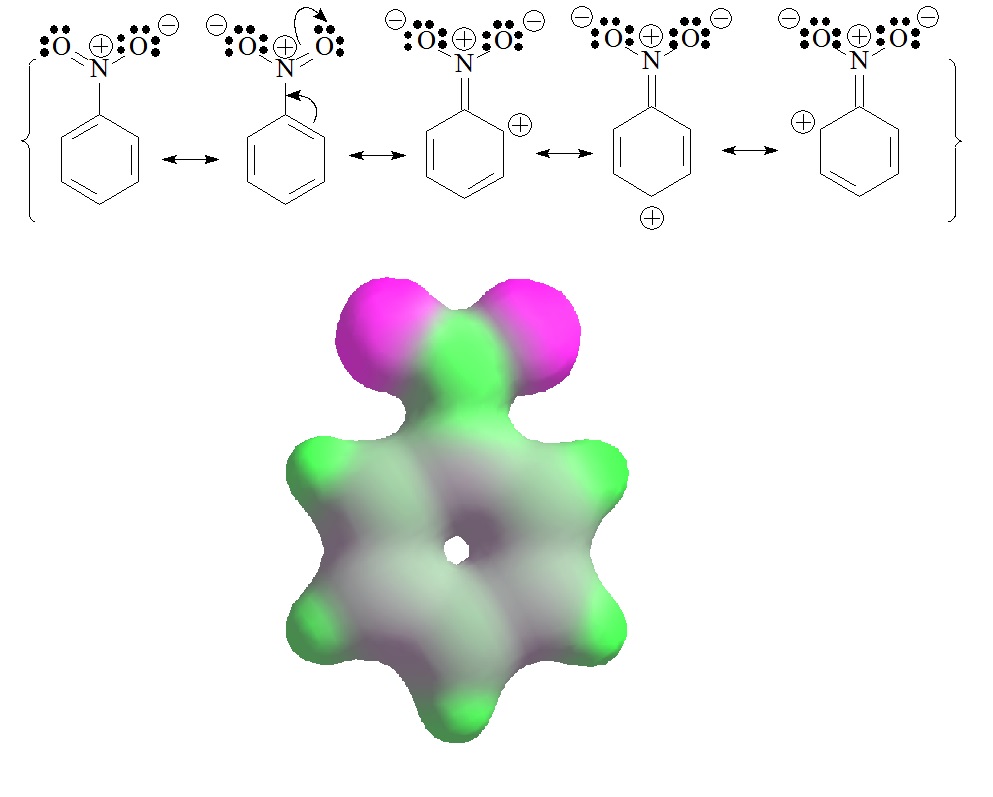
The reality of nitrobenzene is the opposite: the aromatic ring has a slight charge defect in the "ortho" and "para" positions to the nitro group. Five Lewis formulas are needed to explain it.
The predominant forms are the first two, which have the "normal" charge separation due to the structure of the nitro group. The others, although they increase the charge separation, are the only ones that explain why the ring has a slight electron density defect in the "ortho" and "para" positions.


 With this Lewis structure alone the reality of nitromethane cannot be explained
With this Lewis structure alone the reality of nitromethane cannot be explained