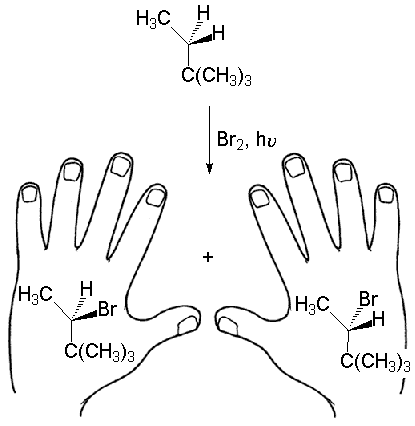
CHIRALITY AND OPTICAL ACTIVITY
In this reaction, is only one product being produced? Or is it two different compounds?
Can you superimpose the two bromoderivatives?
The answer to the last question is NO.
Consequently, the reaction does give a mixture of two different compounds, object and non-superimposable mirror image, i.e. two ENANTIOMERS.
Why two ENANTIOMERS are produced?
Because the two hydrogens that can suffer the reaction are equivalent to each other and have the same chance of being attacked by bromine.
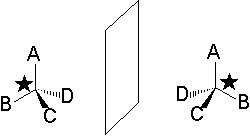
An important class of CHIRAL MOLECULES are those bearing one or more carbons with four different substituents.
Those special carbons are denoted as STEREOGENIC CENTERS or STEREOCENTERS.
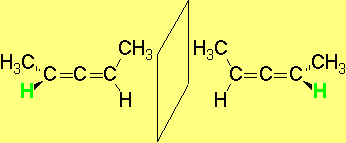
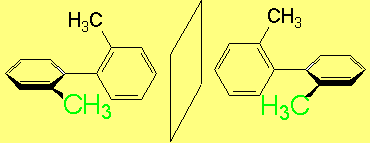
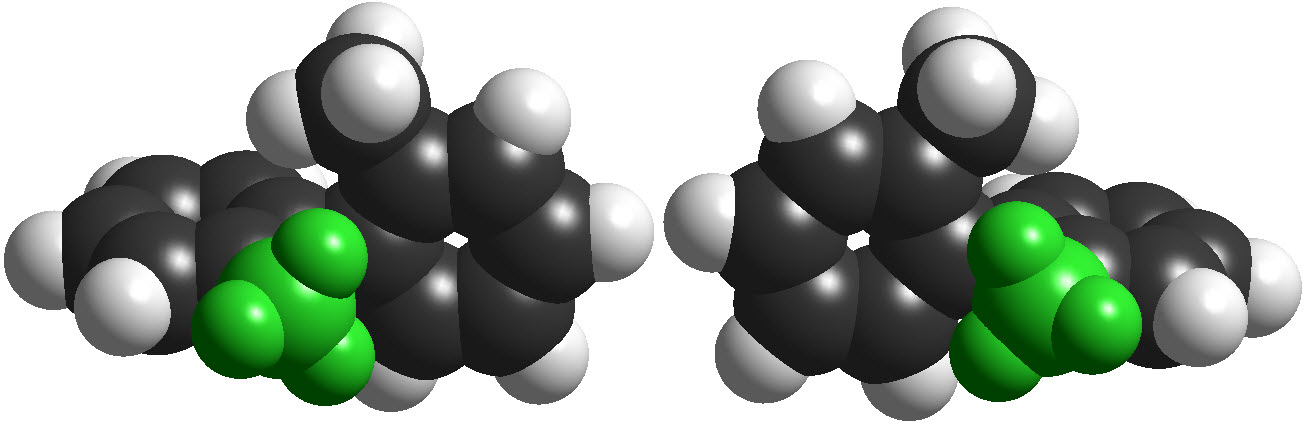
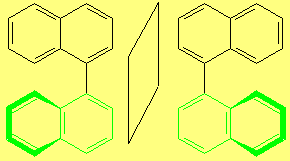
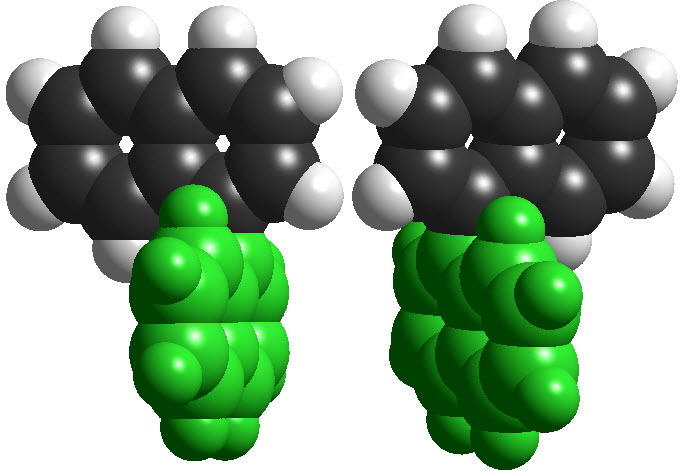
However, to have an even more complex picture of STEROISOMERISM, there exist molecules that are CHIRAL without having any STEREOCENTER.
The most relevant examples are those of ALLENES, BIPHENYLS and BINAPHTHYLS, among many others.
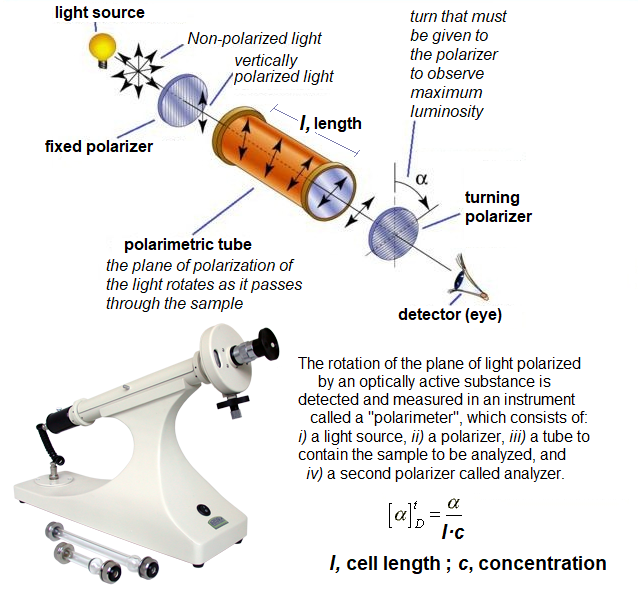
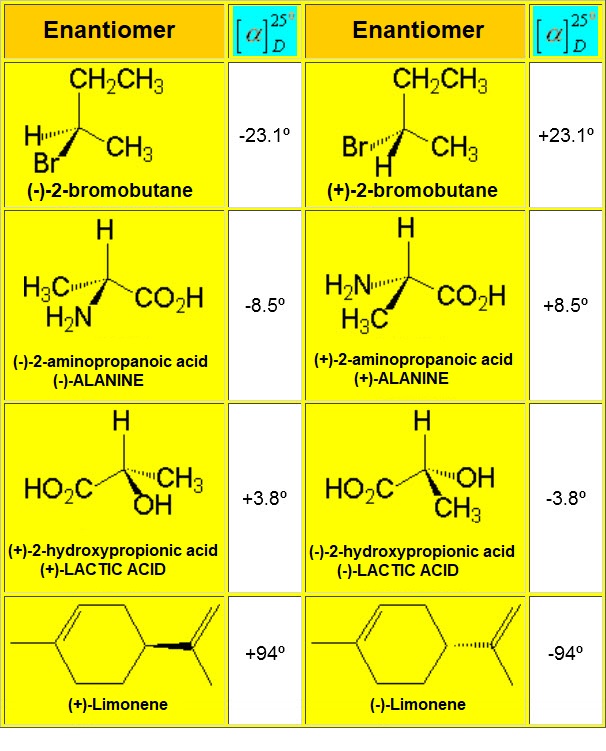
ENANTIOMERS bear the very same physical and chemical properties, importnat exception made of their response to polarized light (OPTICAL ACTIVITY). That's why they are also called OPTICAL ISOMERS.
The specific optical rotation, that is assessed by means of a POLARIMETER, is a physical property characteristic to the structure of each ENANTIOMER, its concentration and the solvent used in the measurement.
The measurement of the specific rotation, when that of each ENATIOMER is known, indicates the ENANTIOMERIC PURITY of the product.
The NON-CHIRAL compounds or the 1:1 mixture of enantiomers (RACEMIC MIXTURES or RACEMATES) are optically INACTIVE and thus give a NULL optical rotation.