In order to transform a carboxylic acid into an ester, one must turn the OH into a good leaving group while in contact with an alcohol that is a weak nucleophile.
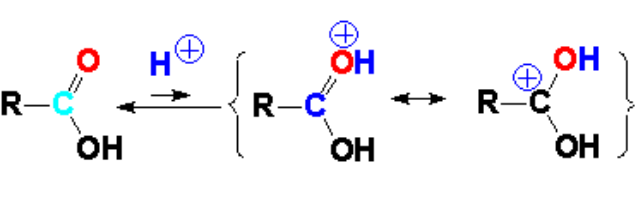
The reaction is carried out with a catalytic amount of a strong mineral acid (hydrochloric or sulfuric acids would do) capable of protonating the weakly basic C=O group.
Protonation of the C=O group increases its carbon's electrophilicity and hence its reactivity towards even weak nucleophiles as, for instance, an alcohol's oxygen.
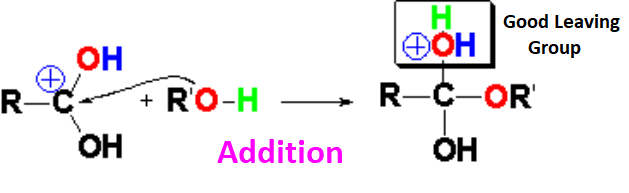
In the presence of an excess of alcohol - usually used as the solvent too - the nucleophilic attack is effected.
One of the two electron lone pairs of the alcohol's oxygen attacks the carboxylic acid molecules that have been protonated by the catalytic amount of mineral acid.
A tetrahedral intermediate is thus formed with an emergent water molecule as an excellent leaving group.
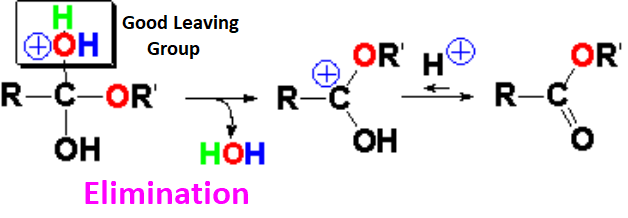
The water loss brings back the protonated structure of the carbonyl group that keeps being linked to the alcohol's oxygen.
Deprotonation leads to the ester and gives back the proton for more molecules of starting carboxylic acid to react.
Ester formation takes place in strong acidic medium, in the presence of an excess of alcohol (solvent) and in the total absence of water.
On the contrary, an ester can be turned (hydrolized) into a carboxylic acid by its treatment with the same acid but in an excess of water.
The mechanism of both reactions is just the same. For ester formation one has to look at it from left to right whereas for ester hydrolyis from right to left (principle of "microscopic reversibilty").