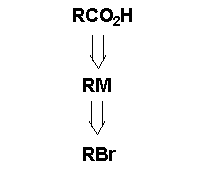
Retrosinthetically speaking, one might plan to have a carboxyl group from a haloderivative, provided the halogen is first replaced by a metal and the resulting organometallic treated with CO2.
Wacht out for the possible incompatibilities among the present functional groups !!!
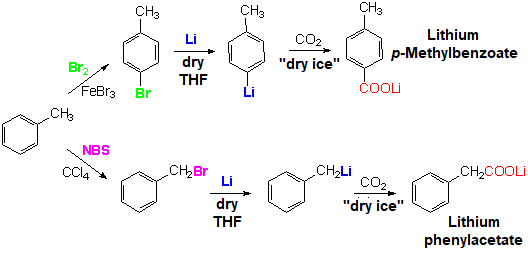
Bromination is instrumental in this Scheme.
If one uses molecular bromine in the presence of a Lewis acid, bromination takes places at the ring by SEAr.
If one chooses in turn N-bromosuccinimide (NBS), precursor of atomic bromine radicals, bromination occurs at the benzylic position.
Metallation - in one case at the ring and in the other at the benzylic position - leads to different organomatallics.
The aromatic organometallic with CO2 yields lithium p-methylbenzoate, whereas the benzylic one renders lithium phenylacetate.
Where I put the halogen, I put the carboxylate!.
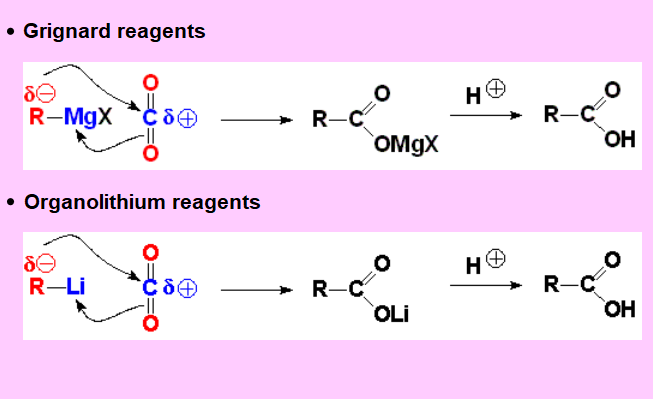 The carbon bonded to the metal, electron rich due to the low electronegativity of the latter, attacks the CO2 carbon, electron defficient because of the high electronegativity of the oxygen atoms.
The carbon bonded to the metal, electron rich due to the low electronegativity of the latter, attacks the CO2 carbon, electron defficient because of the high electronegativity of the oxygen atoms.