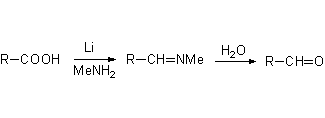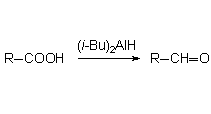REDUCTION OF CARBOXYLIC ACIDS
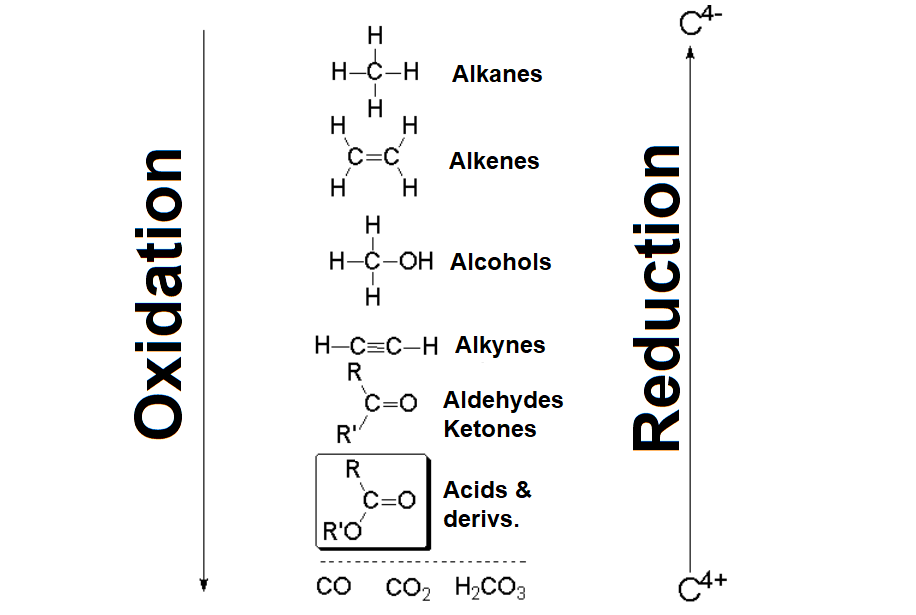
Carboxylic acids and their derivatives bear a formal +3 oxidation state at the C=O carbon. This is the highest possible oxidation state of a carbon in an organic functional group.
Despite that, the oxidation of a carboxylic function can be achieved but always at the cost of degrading the molecule, leading to H2CO3, i.e. CO2 + H2O.
However, a carboxylic acid can be reduced to another functional group with the key carbon in a lower oxidation state, provided there exists the appropriate reagent.
Alkaline metals are excellent reducing agents because of their great tendency to releasing their valence electrons.
The electron-defficient carboxyl carbon can accept an electron and thus reduce. The amine can supply the needed hydrogens.
May you propose a reasonable mechanism?
Diisobutyl-aluminum hydride, much less reactive that lithium aluminum hydride (LAH), partially reduces the carboxyl group ending up in the corresponding aldehyde.
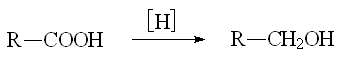
In order to reduce a carboxyl group into an alcohol one must use stronger reducing agents.
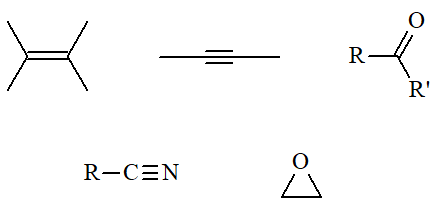
Please, be cautious, other functional groups can be unexpectedly reduced as well !!!
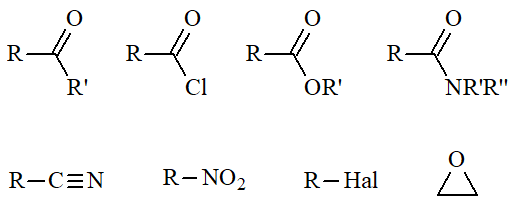
LiALH4 / ether
reduces too:
BH3 / THF
reduces too:
Caution!. LAH reacts too vigorously with a carboxylic acid and nasty explosions and/or fires can easily take place .