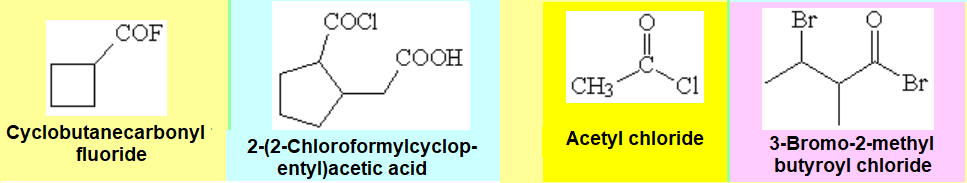
Nomenclature rules are certainly cumbersome and we'll only attemp to name relatively simple molecules. Look at the examples below for representative instances of acid (acyl) halides.
Acid (acyl) halides are formally the condensation between a carboxylic acid and a hydrogen halide (acid + hydrogen halide = acid halide + water).
When the function is principal, they are named as alkanoyl halides and the numbering starts at the carbonyl.
Should the alcanoyl halide be named as a substituent, the term "haloformyl" is then used with the corresponding numeral.