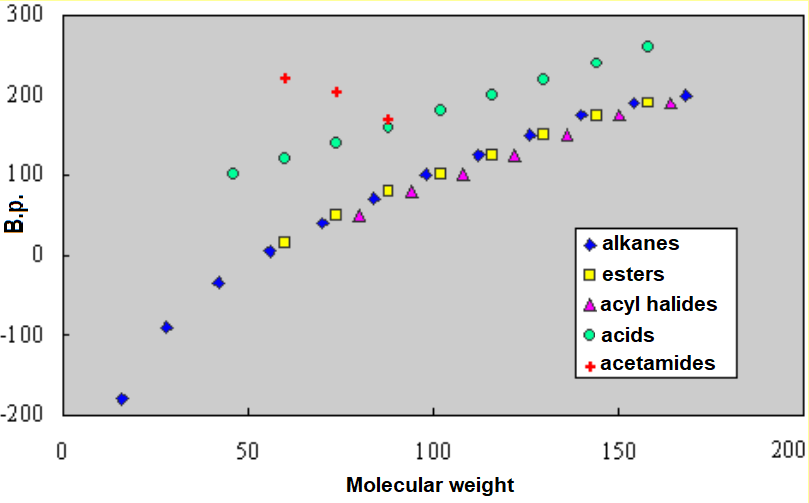
Acetamides have much higher boiling points than the corresponding alkanes of similar molecular weight. For instance, in such a small molecule like acetamide, the polarity of the amide group does contribute to stronger intermolecular forces.
Acetamide, N-methylacetamide and N,N-dimethylacetamide bear boiling points of 220º, 200º and 170ºC, respectively.
Molecular cohesion is higher in acetamide that can accept/give intermolecular hydrogen bonding on its CONH2 group.
N-methylacetamide lacks a hydrogen in its CONH group as compared to acetamide's CONH2. The network of intermolecular hydrogen bonding is less efective in N-methylacetamide thus explaining its lower boiling point.
In N,N-dimethylacetamide there are no hydrogens on N and therefore it cannot establish hydrogen bonding hence displaying the lowest boiling point in the series.