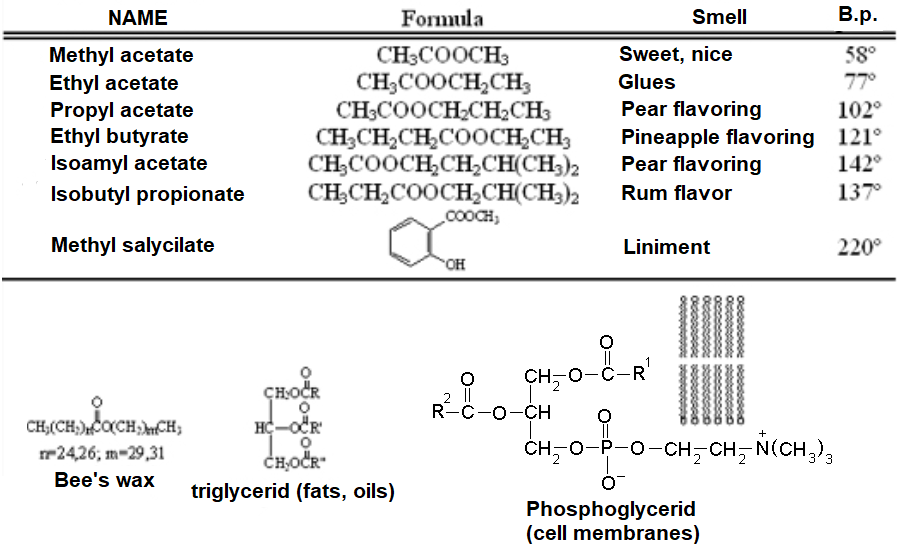
PHYSICAL AND BOND PROPERTIES OF ESTERS
Even though the ester function bears a certain polarity, its presence in a molecule does not make much difference concerning its boiling point as compared to the corresponding alkane, i.e. it doesn't make the intermolecular forces stronger.
Carboxylic acids form dimers and display boiling points equivalent to alkanes of double molecular weight.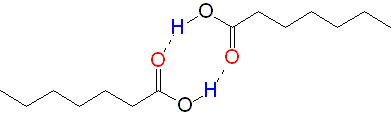 Esters cannot do that.
Esters cannot do that.
Carboxylic acid derivatives can be described by three resonance forms, their relative contribution depending on the nature of L group.
The C=O and C-L bond lengths can be understood by a higher or lower contribution of the first and third resonance forms.
Let's take methanol and acetaldehyde as the reference measurement of true single C-O and double C=O bond lengths.
What conclusion can you draw?
There should be a small contribution of the third resonance form because the single C-O bond in acids and esters is slightly shorter.
Esters are ubiquitous in Nature and essential to life. For instance, the ester function is very important in the molecules building the cell membranes and other natural compounds like for instance fats. Esters are used in everyday life as solvents, flavoring agents and a large etc of other applications.
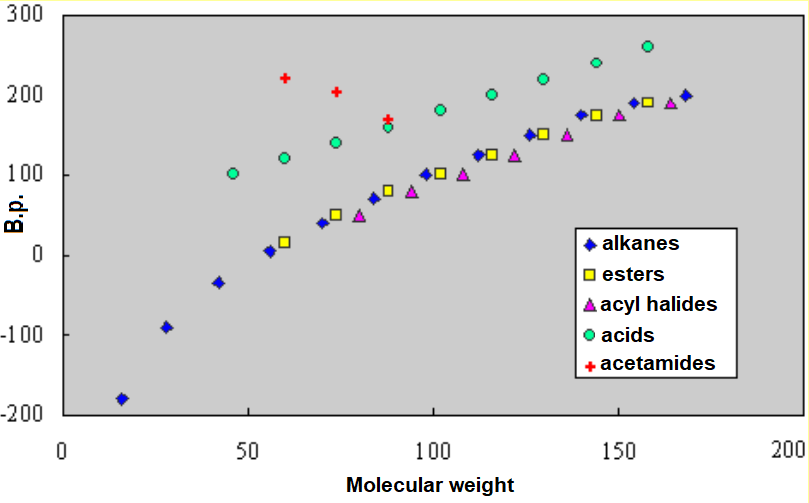
 Esters cannot do that.
Esters cannot do that.