CASE STUDY (cont.)
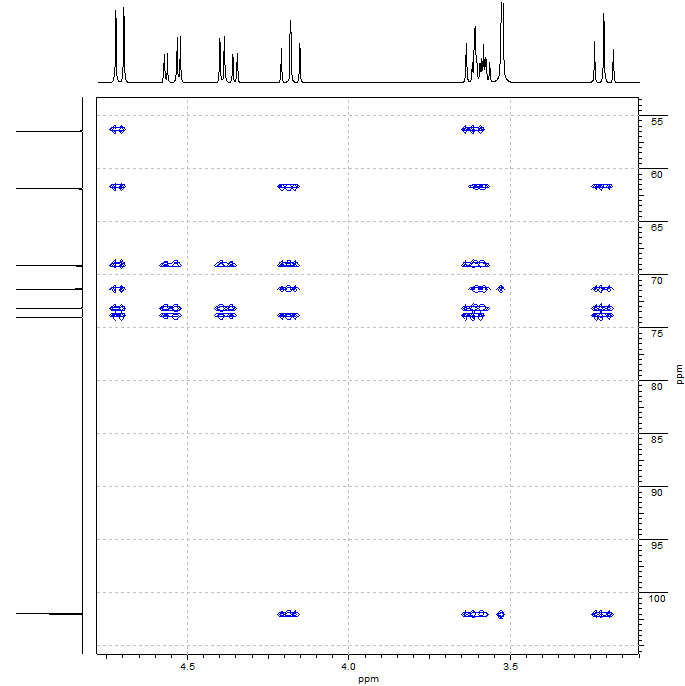
MULTIPLE BOND 2D H/C
In this example the last and definitive spectrum will be presented in order to finally solve the structure of the compound with formula C7H14O6, namely from its multiple-bond 2D H/C correlation.
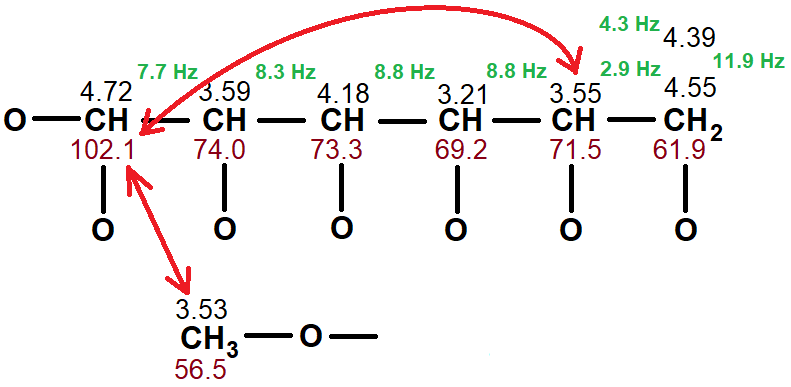
Please, remember that the analysis of the 1D 1H- and 13C-NMR spectra and the 2D COSY H/H and single-bond H/C we deduced this partial structure.
The following facts remains to be ascertained:
1) Which other groups the CH (4.72 / 102.1) is bonded to through its two oxygens.
2) Which other groups the CH2 (4.39, 4.55 / 61.9) is bonded to through its oxygen.
2) Where the OCH3 locates.
Let's look at how the multiple-bond 2D H/C correlation solves these issues:
One of our last unknowns was:
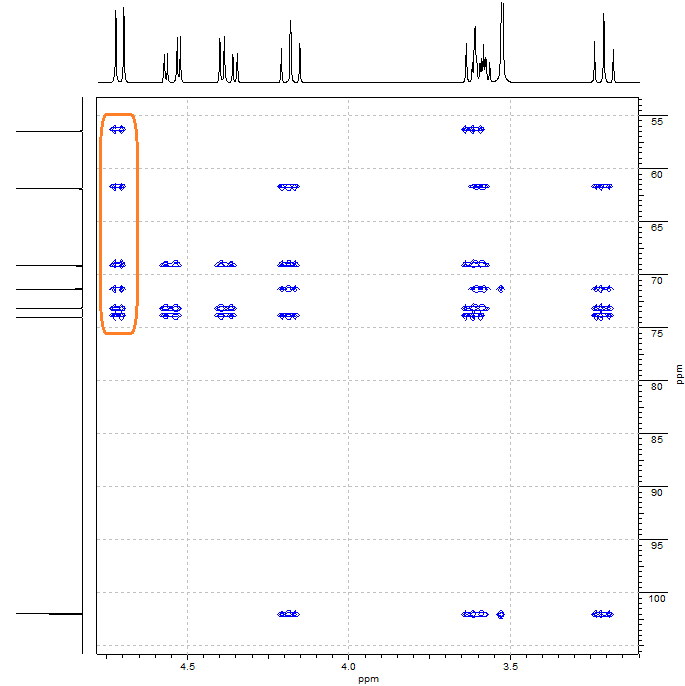
1) Which groups the CH (4.72 / 102.1) is bonded to.
We ought to search for correlations between the 1H at 4.72 and some 13C signal; and between the 13C at 102.1 and some 1H signal.
The 1H at 4.72 ppm displays correlations with all of the six carbons to which is not directly bonded.
Does this fact help us in any way?
I think so!
Look at the fragment figured out for the time being. The 1H at 4.72 ppm is at a distance of 5 and 6 bonds, respectively, from the 13C'sat 71.5 ppm and 61.9 ppm, too far away to explain their correlation.
What if the CH (4.72 / 102.1) would be bonded by an oxygen to the CH2 group?
The distance would thus be reduced to 3 and 4 bonds, respectively, from the 13C'sat 61.9 ppm and 71.5 ppm.
We can double-check this by observing the correlations shown by the 13C at 102.1 ppm.
It correlates with the 1H'sat 4.18, 3.59, 3.55, 3.53 and 3.21 ppm.
The most relevant ones have been marked in red on the spectrum and with red arrows in the structure.
The correlations of the 13C at 102.1 with the 1H's at 4.18 (3 bonds), 3.59 (2 bonds) and 3.21 ppm (4 bonds) were expected from the structure deduced from previous spectra.
However:
1) Its correlation with the CH at 3.55 ppm cannot be attained through 5 bonds. The presence of this correlation makes us think that this CH (3.55 ppm) must be bonded to the 13C at 102.1 ppm through an oxygen, therefore reducing the distance between them to 3 bonds.
2) Its correlation with the OCH3 (3.53 ppm) strongly suggests that the CH (4.72/102.1) and the OCH3 must be bonded each other for them to be close enough to one another (3 bonds).
In summary, our final guess about the structure is as follows, placing the four remaining hydrogens:
As if it were magic, the structure ends up being a methoxy derivative of an hexose. The high interproton coupling constants between the vicinal 1H's are compatible with their axial arrangement and hence with the structure of beta-2-methoxyglucose.