SIGNAL SHAPE
(COMPLEX COUPLING)
Sometimes the neighboring 1H's to other(s) don't join forces and the produced signal splittings DO NOT obbey the N+1 rule, nor can their intensity be predicted by Tartaglia's triangle...
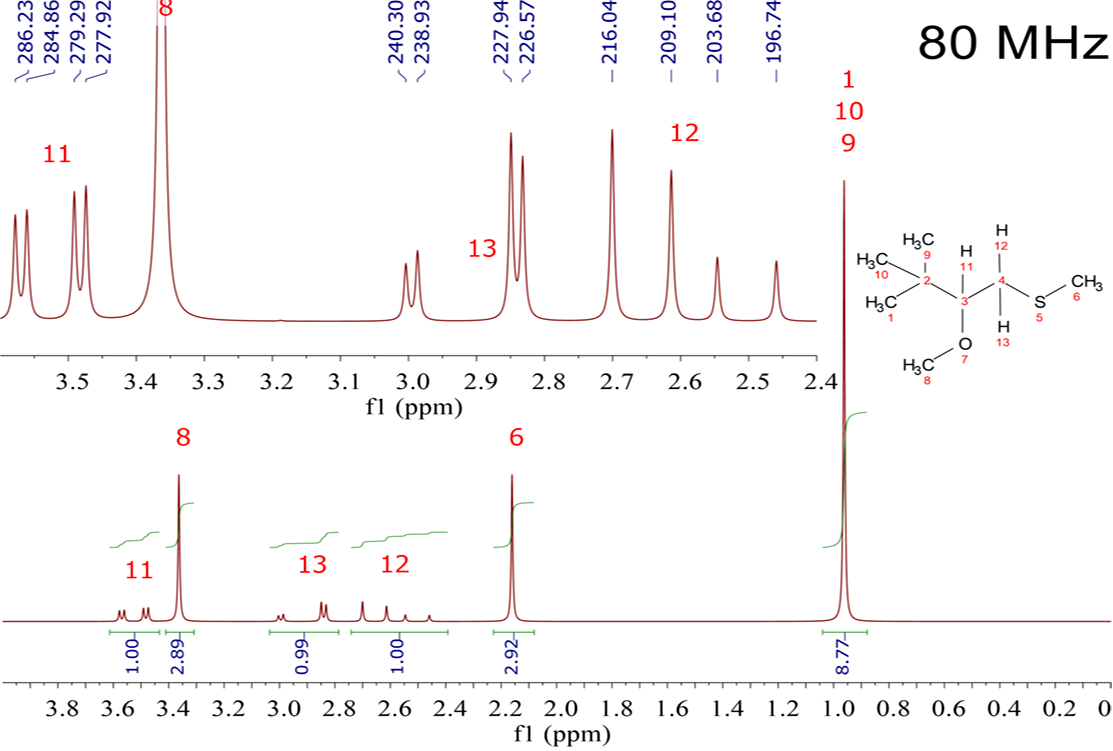
Please, look attentively to the following spectrum. It belongs to the indicated structure (CH3)3CH(OCH3)CH2SCH3. Signals are assigned with the red numbering.
The easiest signals to understand are the singlets with the 3:3:9 area (integral) which belong to the various CH3 groups.
The three CH3 groups with numerals 1, 9 and 10 are equivalent and resonate together. They don't have any neighbor and are bonded to [C(2)] of low electronegativity. That explains their low chemical shift (0.95 ppm).
The two CH3 with 6 and 8 numbering are clearly non-equivalent, neither between them, nor regarding those of the C(CH3)3 group. That's why they give two different singlets because, besides, they don't have neighbors.
Electronegativity order is O > S > C. Because of that, 1H's of CH3O are the most electron deshielded and appear at the highest chemical shift (3.35 ppm), followed by those of CH3S (2.15 ppm) and those of C(CH3)3 (0.95 ppm).
As in many other 1H-NMR spectra, the great difficulty is the understanding of the signals belonging to H(11), H(12) and H(13). Let's reflect a bit.
H(11) bears two neighbors, H(12) and H(13) (N=2). How would it show up? As a triplet (N+1)?
Unfortunately, IT ISN'T A TRIPLET AT ALL. It is a "double-doublet".
On their part H(12) and H(13) look equivalent at first sight and should resonate together as a doublet because of their only neighboring H(11). However, they don't!!!
Both, H(12) and H(13), show up as "double-doublets" with weird "leg" intensities.
Can we understand all that? Yes, of course.
We actually did make a mistake in considering H(12) and H(13) as equivalent. Why aren't they equivalent?
The key to this mistery is C(3): it's got four different substituents and therefore is a "stereocenter", i.e. a disymmetry-generating center.
The two hidrogens of CH2 groups are in general equivalent exception made when the molecule bears a "stereocenter".
The presence of such a "stereocenter" breaks the symmetry.
The two 1H's of any CH2 group in a molecule bearing at least one "stereocenter" stop being symmetry-equivalent and can resonate at different chemical shifts, as it happens with H(12) and H(13) in the molecule under scrutiny.
H(11), H(12) and H(13) influence one another but in an individual manner because any possible equivalence among them is broken by the presence of the sterocenter at C(3).
Had there been symmetry, H(11) would have been influenced by H(12) and H(13) together and H(11) would have showed up as a triplet (N+1 rule). However, since the symmetry is broken, H(12) and H(13) don't join forces and influence H(11) individualy, each of them in a different way, giving rise to two different splittings, i.e. a double-doublet.
The signal splittings are measured in Hz and are:
J3(H11/H13) =1.37 Hz
J3(H11/H13) = 6.96 Hz
The influence (coupling) is always reciprocal and H(12) and (H13) signals must contain those very same couplings of 1.37 Hz [seen in H(13)] and 6.96 Hz [seen in H(12)].
Where does the 12.36 Hz splitting come from? You guessed it!!!
From the reciprocal influence the non-equivalent H(12) and H(13) exerts to each other, caused by the symmetry-breaking "stereocenter" at C(3).
In this case notation is:
J2(H12/H13) = 12.36
J2 and J3 stand for two- or three-bond couplings, respectively.