PHYSICAL BASIS OF CHEMICAL SHIFT IN 1H-NMR
The different 1H nuclei (or 13C) belonging to a given molecule, do they all "suffer" the same magnetic field?
You already know the answer, yet the question has been asked in an unexpected way.
Since the different 1H nuclei (or 13C) within a molecule DO NOT resonate at the same frequency, the answer to this question must be a rotund NO.
THE 1H NUCLEI (or 13C) WITHIN A MOLECULE DO RESONATE AT DIFFERENT FREQUENCIES AND, THEREFORE, THEY MUST "FEEL" SLIGHTLY DIFFERENT MAGNETIC FIELDS.
If the external magnetic field is fixed, how come the active nuclei don't suffer it in the same way?
The answer is in the electron density surrounding the nuclei.
Do all 1H or 13C within a molecule have the same electron density?
With a minimum amount of knowledge about Organic Chemistry, you must say NO.
What's the reason for the nuclei to have more or less electron density around?
Put the blame on the neighboring nuclei and their ELECTRONEGATIVITY.
The concept of ELECTRONEGATIVITY is very important in Chemistry: It is the capacity of an atom to withdraw electron density from the bond(s) it sustains with (an)other atom(s).
Let's get back to our recent example: CH3CHClCH3.
The Cl is quite electronegative because it bears 17 protons at its nucleus that strongly attract the electrons from its C-Cl bond.
The CH ends up with a heavily reduced electron density.
On their part, the 13C and 1H of the CH3 groups, two and three bonds apart from Cl, endure a lower reduction of their electron density because of the larger distance.
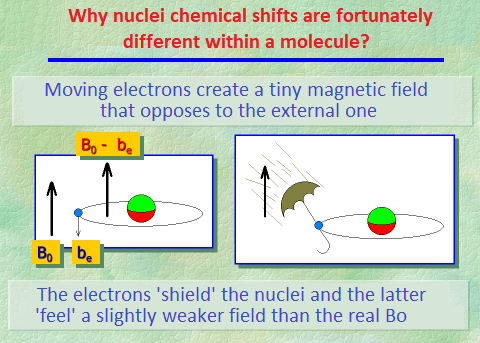
Electrons are charged particles spinning around the nuclei and thus create tiny magnetic fields opposing to the external one of the NMR instrument.
The lower the electron density around the 1H or 13C nuclei, the higher the external magnetic field they "feel" and the higher the frequency at which they "resonate".
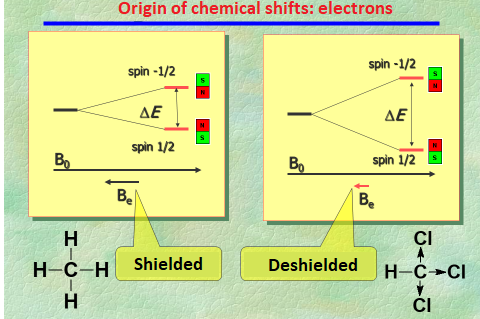
In short, electron density "shields" the nuclei from the external magnetic field.
The various 1H or 13C, depending on their location at a given molecule, will find themselves more or less shielded, thus showing up at different "chemical shifts".
It is this "shielding" phenomenon the one that makes NMR such a powerful tool to figure out organic structures.
Let's go back once again to our example and use the typical jargon used in NMR:
The molecule CH3CHClCH3 renders two signals in the 1H-NMR spectrum, at 3.97 ppm and 1.55 ppm, which are assigned to the CH and CH3 groups respectively.
The CH's 1H is at a "more deshielded" chemical shift than the CH3 groups' as a result of the Cl atom's electronegativity (electron acceptor by inductive effect).
Summing up, nuclei are the main characters in the "NMR movie" but their electronic "clothes" make them go "stage right or left".
A light electron cloud means a lower shielding and a higher chemical shift ("stage left").
A dense electron cloud means a higher shielding and a lower chemical shift ("stage right").
In general, the heavier or lighter electron "clothing" of the 1H (or 13C) nuclei depends on their larger or shorter distance to electronegative atoms and/or the quantity of the latter.
The series CH4, CH3Cl, CH2Cl2 y CHCl3, that we studied in the previous section, is a good example of quantity.
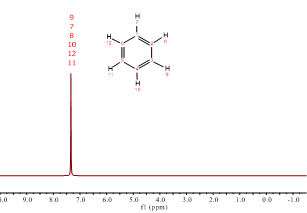
But look at the following spectra.
Isn't it puzzling? Why benzene's 1H's resonate at chloroform's chemical shift? Is there any electronegative atom in benzene?
Good questions indeed!!!
Of course, benzene bears no electronegative atom.
The six electrons of benzene's aromatic cloud are responsible.
Please, recall what an aromatic ring is and its electron properties.
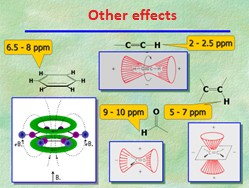
Aromaticity implies heavy electron delocalization. Electrons are literally circulating over benzene's hexagon and, as usual, they create a magnetic field that in this case reinforces that of the NMR magnet exactly at the location where 1H's are placed.
The location of benzene's 1H's at the edges of the ring makes them "feel" the external magnetic field plus the electron's thus resonating at higher frequencies without the need of any electronegative atom.
In general, multiple bonds exert this type of effect and the bonded 1H's show up at unexpectedly high chemical shifts, like benzene's.
Following this, aldehyde's CHO displays an extremely high chemical shift because of the combined effect of the double C=O bond and oxygen's electronegativity.
In summary, 1H nuclei show chemical shifts conditioned by the electronegativity of the neighboring atoms and by possible electron currents taking place at multiple bonds.
The graph shows the expected chemical shift regions for a bunch of functional groups.
THe 1H chemical shift can span up to 15 ppm but, in general, the usual range is 0-5 ppm or 5-8 ppm for 1H's bonded to sp3 or sp2 carbons, respectively.