SIGNAL INTENSITY IN
13C-NMR
The signal areas in 13C-NMR IS NOT EXACTLY PROPORTIONAL to the number of 13C's responsible of them as it was in 1H-NMR.
You may see this clearly in the two following spectra:
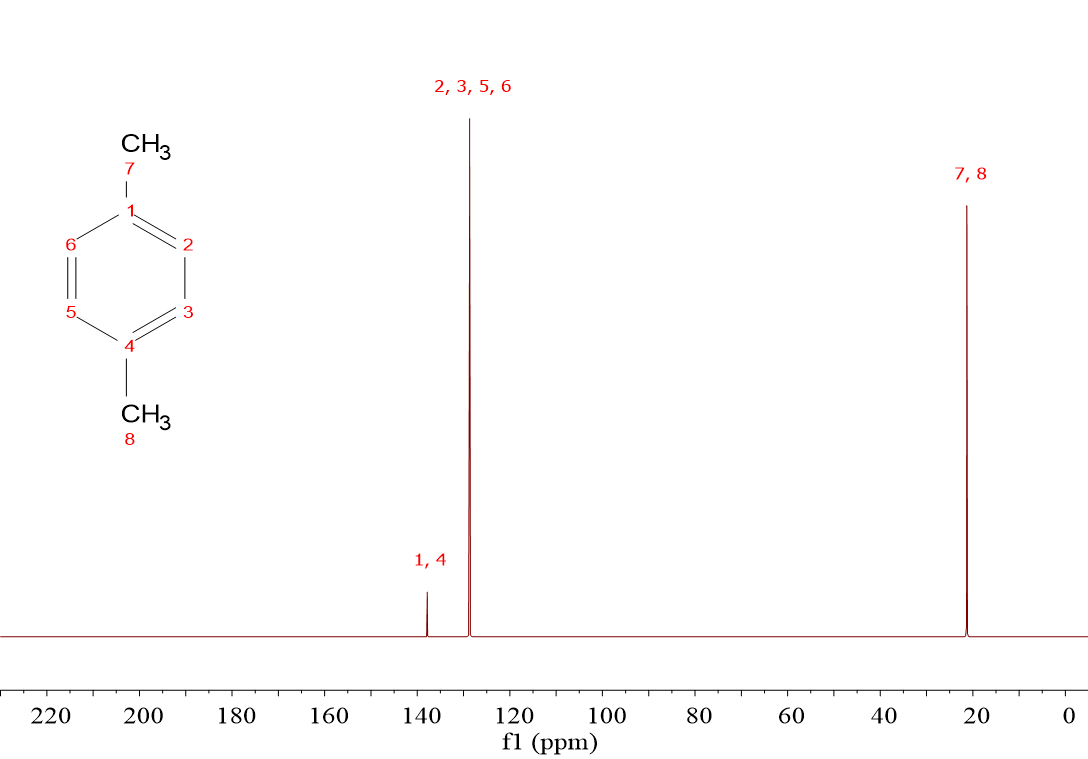
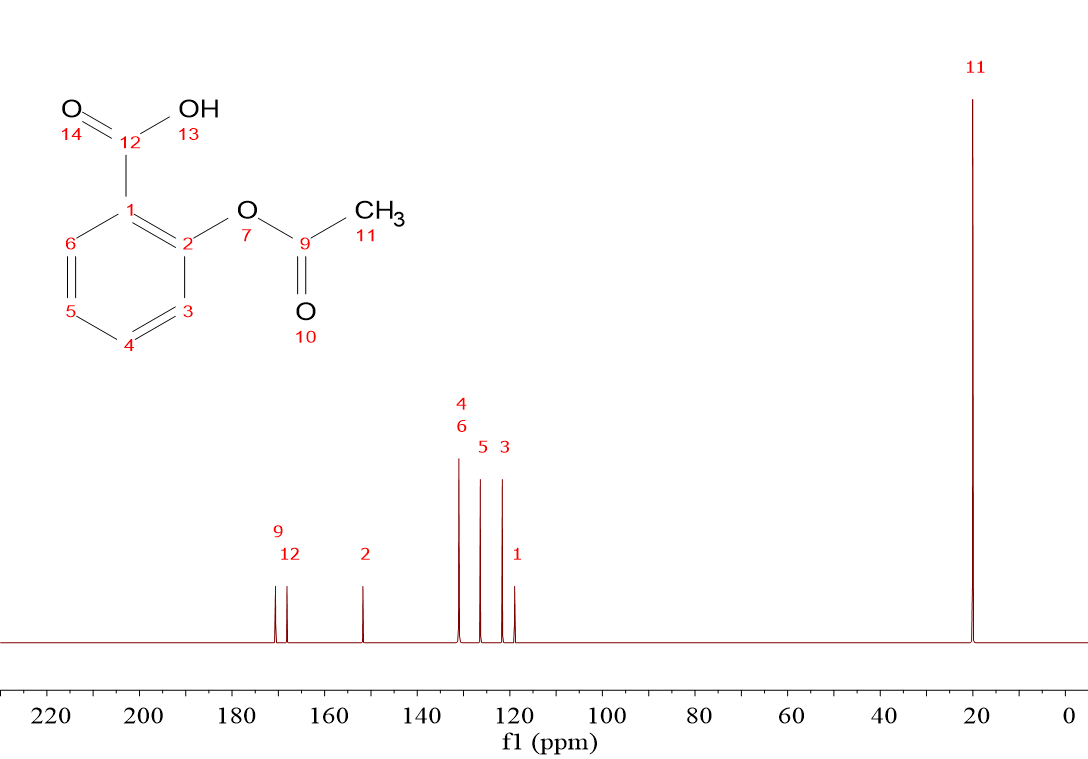
It is immediately evident to the naked eye that the “non-hydrogenated” 13C's display a significantly low intensity.
This is the case of C(1) and C(4) on the left spectrum (1,4-dimethylbenzene) and C(1), C(2), C(9) and C(12) on the right spectrum (acetylsalicylic acid or “aspirin”).
Let me explain that with the lowest possible theory level.
The NMR spectra come from the observation of how nuclei release the energy gained from the radiofrequency in the magnetic field.
The excited nuclei lose their energy by passing it to neighboring NMR-active nuclei.
In the case of 1H's, there are almost always other neighboring 1H's ready to accept the released energy.
However, in the case of the 1.1% of 13C in a molecule, 13C's cannot release the energy to neighboring carbons that 98.9% of them are NMR-non-active 12C.
13C's can only pass their gained energy to neighboring 1H's.
For non-hydrogenated 13C's, their energy release is much more difficult and, because of that, they display very low intense signals.
In the NMR process, nuclei release the gained energy from their interaction with the radiofrequency within the magnetic field, but it takes some time ("relaxation time").
In 1H-NMR, 1H's have in general similar and relatively short "relaxation times".
All 1H's within a molecule release their gained energy in a similar time lapse and the intensity of their signals (area or integral) corresponds to the number of 1H's responsible of them.
However, in 13C-NMR there usually are 13C's with specially long "relaxation times" and their signals become much less intense.
This is the case of the "non-hydrogenated" 13C's, i.e. not directly bonded to 1H as it happens in carbonyl groups, exception made of aldehydes.