SIGNAL INTENSITIES
(INTEGRAL)
The area of the signals in 1H-NMR or, in other words, their “integral”, corresponds to the number of 1H's responsible of them.
Look at the following spectra:
Assume that you don't know the structures.
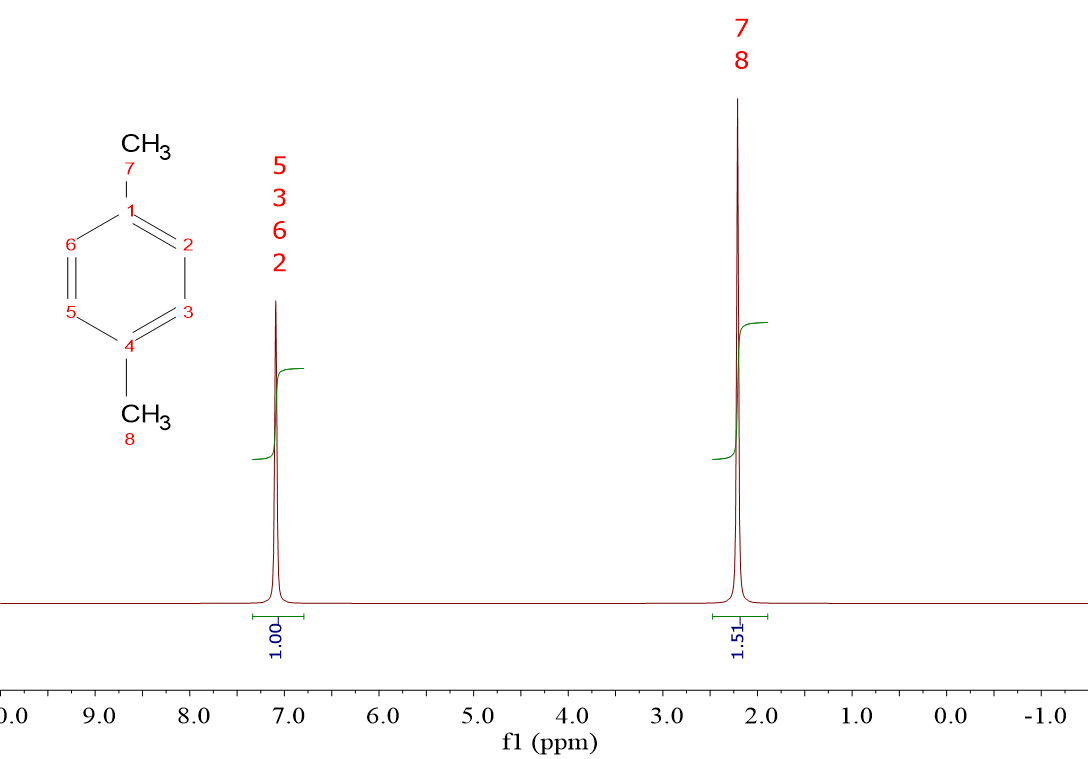
Look at the left spectrum. It has been integrated assigning unity to the smallest integral.
Does the other integral (1.5) make any sense?
Is it posible to have one 1H and "a half"?
You guessed it!!! Of course not!!!
In order to make sense of it, one can multiply the integral numbers by integers, i.e. 2 or 4, etc.
With that simple maneuver, integral ratios of 2:3 or 4:6 etc. can be reached with integer number of 1H's.
Look now at the structure. The signal at the highest chemical shift (7.1 ppm) must belong to the 1H's directly bonded to the ring, because they suffer the direct deshielding of the circulating electrons in the cycle.
They are four and equivalent by symmetry.
The other signal must belong to the six 1H's of the two CH3 groups which are equivalent too.
Consequently, the real integral ratio must be 4:6 and the multiplication by 4 has been necessary.
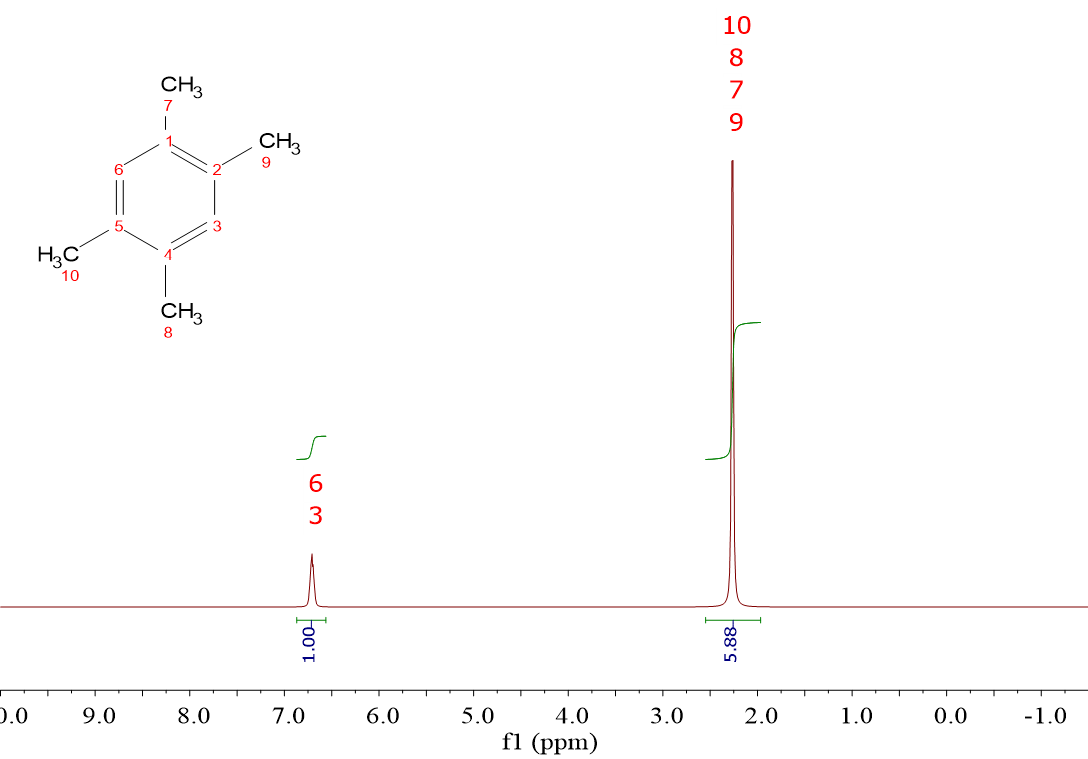
In this spectrum the real integral ratio is 2:12. Agreed?
However, if we multiply by 2, the right hand side signal ends up with an integral of 11.76, not an integer number.
Please, consider that integrals are not 100% precise.
Integration is a graphical operation bearing an intrinsecal error that varies from 5-10% depending on the spectrum "noise" and the proximity of other signals.
Summing up, in the integration proces, unity must be assigned to the smallest integral. One then multiply all integrals by the appropriate integer number, in order to get all of them as closer to integer numbers as possible.
Don't forget the integral errors that can reach even 10% and not uniformly across the spectrum.
Look at these two new examples and learn more "tricks".
At a once-over glance, they look very much alike but there's a big difference in the solvent used to dissolve the sample.
In this case D2O, i.e, deuterated water”.
In this case CDCl3, i.e, “deuterated chloroform”.
First things first! One must locate the solvent signals.
Solvent signals do not belong to your structure and must not be considered in the structural elucidation. Yet one must recognize them.
Save very rare exceptions, INTEGRATION OF SOLVENT SIGNALS MAKES NO SENSE AT ALL.
You have noticed that NMR solvents are usually deuterated ones. They don't have hydrogen (1H) but deuterium (2H), hydrogen's isotope with one neutron at the nucleus.
No doubt the solvent is the most abundant species in a NMR sample and therefore, had it not been deuterated, it would give a huge signal obscuring the observation of our molecule.
DEUTERIUM (2H) IS NMR ACTIVE BUT IT RESONATES AT A DIFFERENT FRECUENCY TO THAT OF 1H, because 2H and 1H bear very different "gyromagnetic constants" despite their similarity.
Due to that, 2H does not show up in the 1H-NMR spectra. Nevertheless, a small solvent signal is always seen that corresponds to the small amount of the residual 1H that deuterated solvents always carry, regardless of their purity and pricing.
Excluding the solvent signals (4.79 ppm and 7.26 ppm for the residual H2O and CHCl3, respectively), spectra are very much alike.
Signal shapes (“quartet” and “triplet”) and integral ratios are almost identical.
However, chemical shifts are slightly different: 3.8 ppm and 1.3 ppm, left hand side spectrum; 3.5 ppm and 1.2 ppm, right hand side spectrum). Will they be the same compound?
To tell you the truth, I wouldn't bet...
ETANOL
(CH3CH2OH).
DIETILÉTER (CH3CH2OCH2CH3).
Even though they had been the same compound, the different solvent used may induce slight chemical shift variations, similar to those observed for the two different structures.
That's why I did advise you not to bet on this...
How would we solve the problem?
NMR is extraordinary but there's life beyond it!!!
Other techniques may come handy to help us.
For instance, an IR of the two pure substances would have told apart the two functional groups, wouldn't it?
A mass spectrum would have given us the answer as well because the two compounds have very different molecular masses...