The name Nuclear Magnetic Resonance (NMR) tells us that it should consist of a technique related to the atomic nuclei.
But, don't worry!!!
It has nothing to do with radioactivity, something that the word "NUCLEAR" always suggests to the lay man.
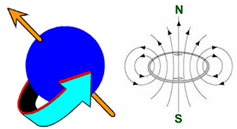 Schematic representation of nuclear spin.
Schematic representation of nuclear spin.
In NMR spectroscopy, atomic nuclei are the main stars and the electrons behave as supporting actors, although the role of the latter is indirectly crucial.
Many isotopes, from the large amount of them in the Periodical System, have nuclei bearing magnetic properties: they behave as tiny magnets.
The most simple example is the proton, i.e. the hydrogen nucleus (1H), with a natural abundance of nearly 100%.
Another important example is the carbon isotope 13 (13C) of mass 13.003, possessing 6 protons and 7 neutrons, whose abundance is only slightly above 1%. Low but tangible an abundance!!!
Which are the most abundant and most important elements to an organic molecule?
Hydrogen and Carbon, respectively, aren't they?
Wouldn't it be great to receive a signal from every carbon and hydrogen of an organic molecule?
In addition to that, if we only were able to cleverly process that information, we would literaly get a complete map of the molecular structure.
Believe it or not, that extraordinary task is achieved by our recording of NMR spectra!!!
All subatomic particles, protons and neutrons, bear a property named after the word "spin", kind of twisting around, isn't it?.
A nucleus is in fact a charged particle. It happens that, if the nucleus is constituted by an odd number of protons, an odd number of neutrons, or an odd number of both, like in 1H or 13C (not in 12C!!!), the spin in excess of the odd particle in excess creates a magnetic field that makes the nucleus behave as a tiny magnet.
Organic molecules bear numerous tiny magnets along their structures because 100% of the 1H and 1% of the 13C behave like that.
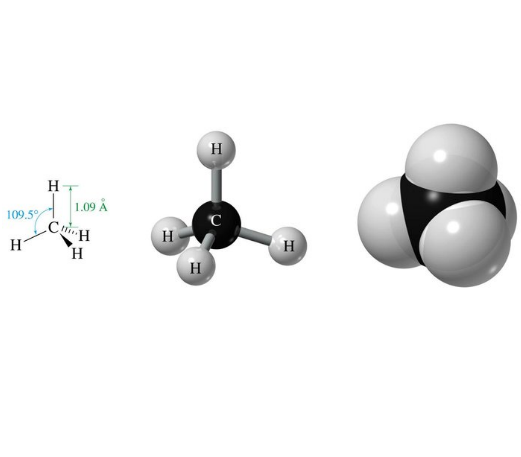
Consider now the simplest organic molecule: methane (CH4).
It is made from one carbon, from which 1% is 13C, and four 100% 1H hydrogens.
The molecule is totally symmetric and the four hydrogens are fully spatially equivalent and hence indistinguishable.
We thus have five magnets, four equivalent ones (4 x 1H) and another different (1 x 13C).
But reflect and think slighly different!!!
Methane is composed of two different kinds of molecules:
A 99% of 12C(1H)4 and 1% de 13C(1H)4.
The predominant molecules 12C(1H)4 bear only four equivalent magnets because 12C, with an even number of protons (6) and neutrons (6), does not have magnetic moment due to the evenness of the particles that cancels out the overall "spin".
However, 1% of the molecules 13C(1H)4 do have the five magnets, four equivalent 1H and a single 13C.
Please, do keep this reflection in mind for future chapters.
 Schematic representation of nuclear spin.
Schematic representation of nuclear spin.