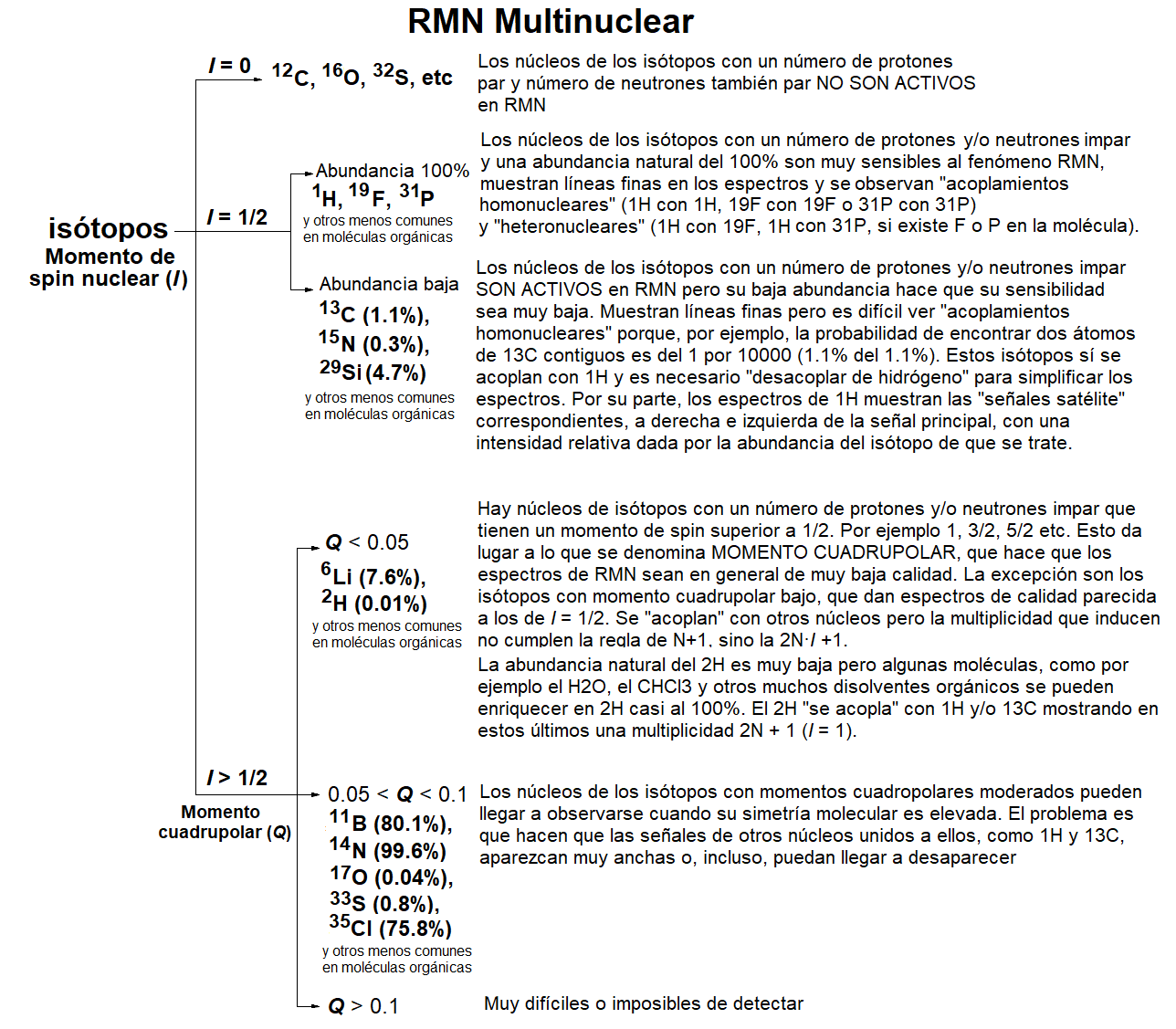
Practically all elements of the Periodical System have at least one NMR-active isotope.
The most common ones in Organic Chemistry are of course 1H (100% abundant) and 13C (1.1% abundant).
Organic molecules frequently bear fluorine and or phosphorous.
Both 19F and 31P are 100% abundant NMR-active isotopes.
The presence of NMR-active isotopes influences 1H's and 13C's and their existence can be inderectly deduced by the changes they cause in the 1H- and 13C-NMR spectra.
The majority of the NMR-active isotopes, bearing "nuclear spin momentum", usually have as well what is called "quadrupolar momentum" which precludes their observation in NMR.
Fortunately, that's not the case for 1H, 13C, 19F, 31P and some others.