SOME PREVIOUS EXAMPLES
(13C-NMR)
Let's start deciphering spectra...
But, let me first remind you of a crucial fact:
in the case of 13C-NMR the active isotope is 13C whose natural abundance is 1.1%.
This makes a big difference because 98.9% of the total content of carbon is useless as 12C is not NMR activeat all.
This heavily affects sensitivity and makes necessary the use of more sample and/or longer recording time as compared to 1H-NMR.
However, 13C-NMR is today routine...
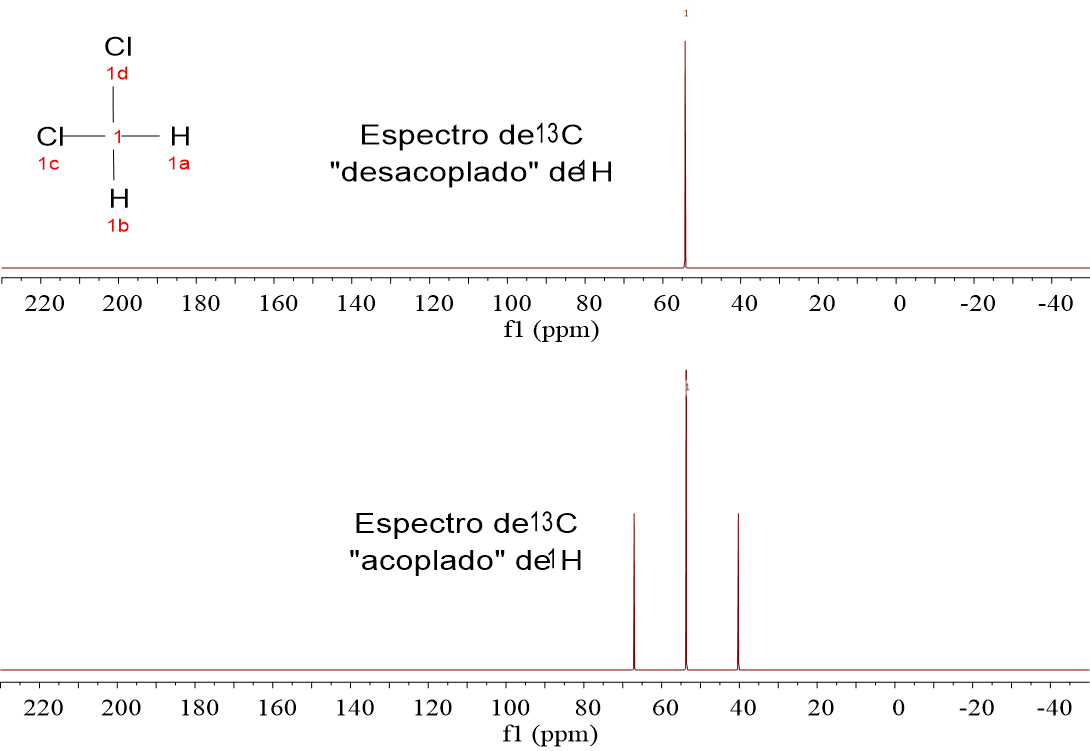
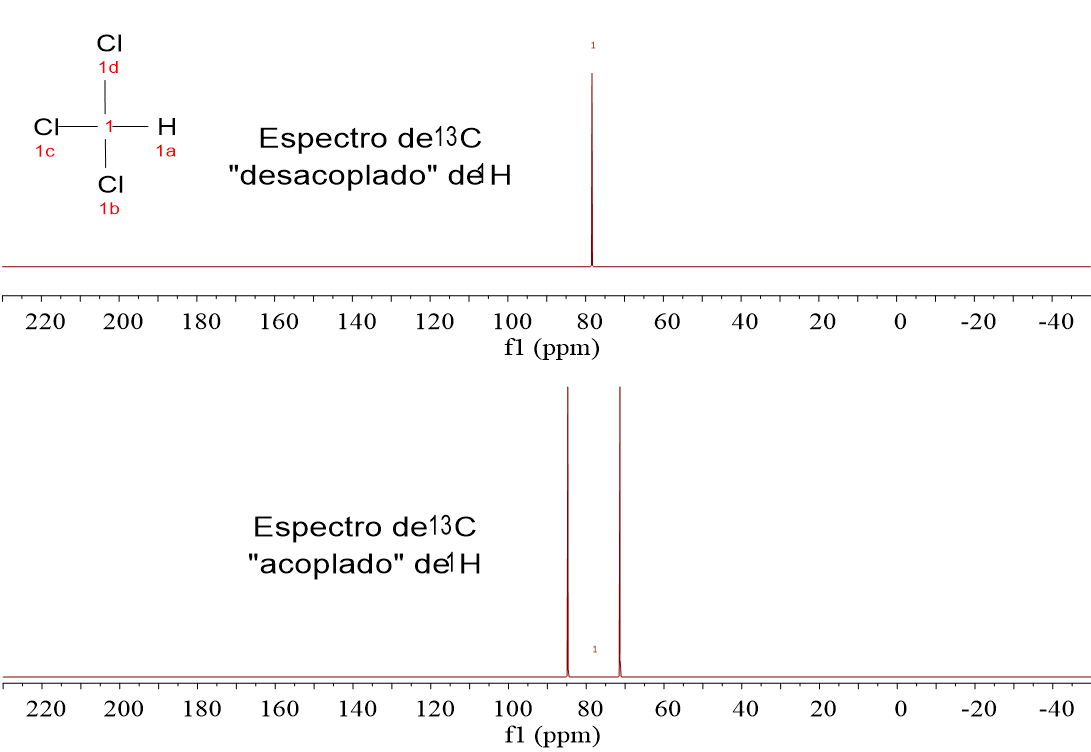
Look at these four 1H-NMR spectra. They belong to the four very simple alike molecules already analyzed by 1H-NMR:
CH4, CH3Cl, CH2Cl2 and CHCl3.
What do you see?
We have recorded two spectra for each substance. Those at the bottom have been registered without doing anything special.
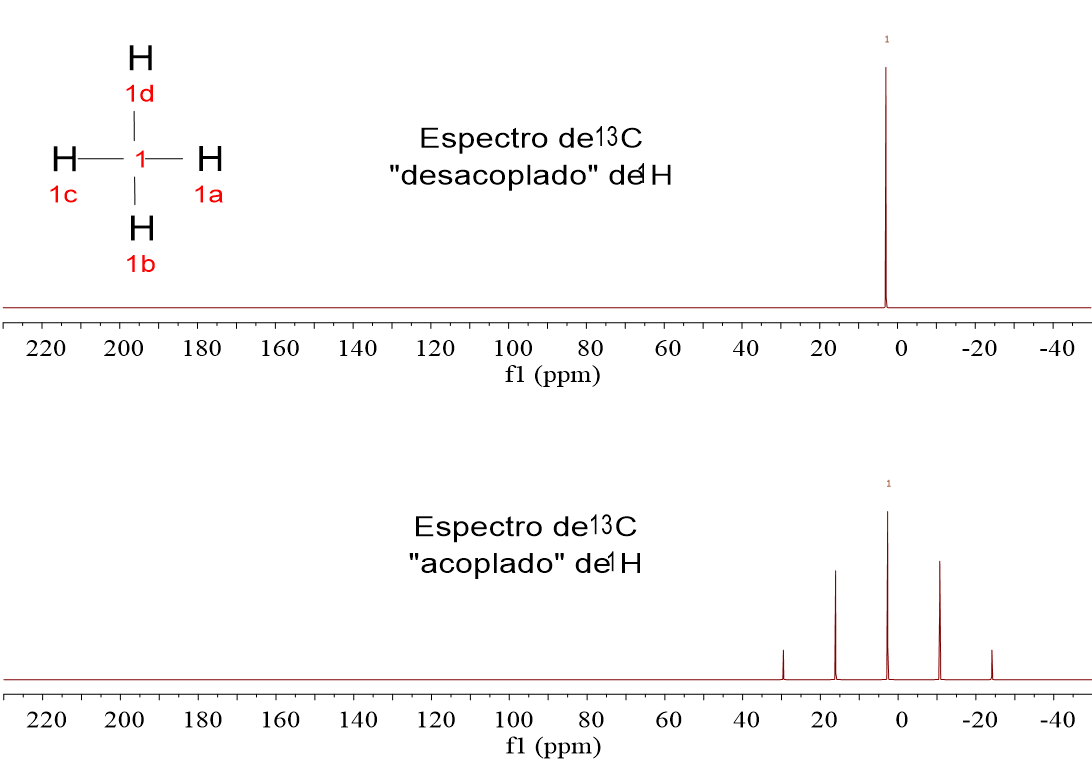
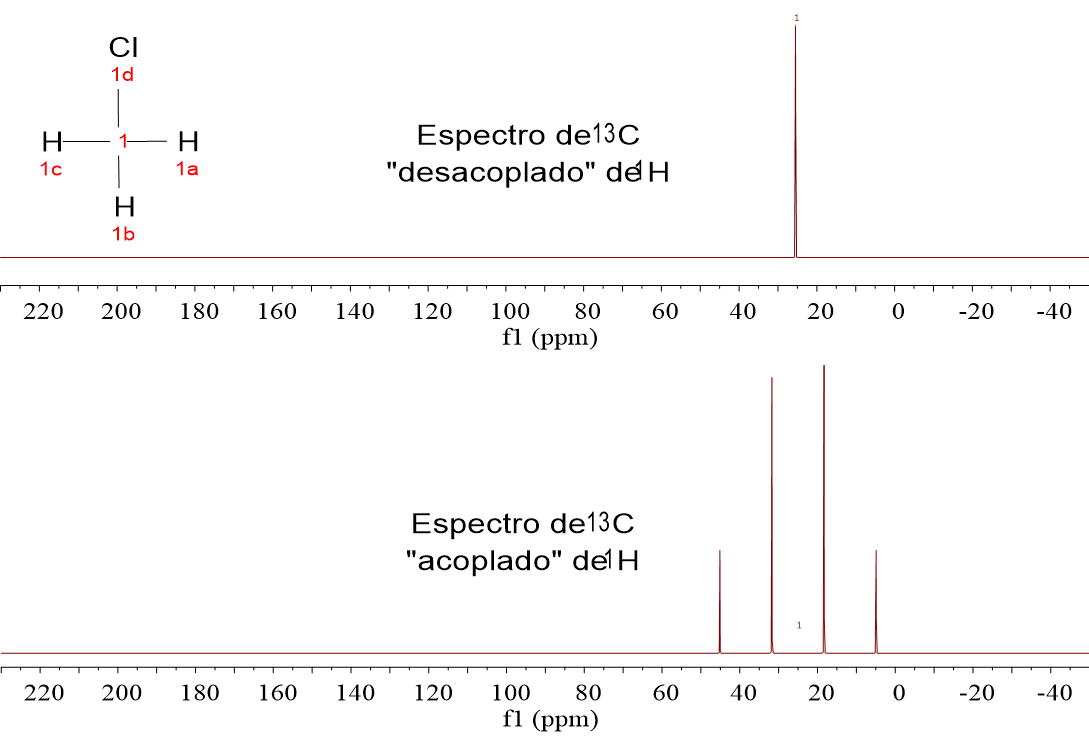
The four substances display one single signal because they only bear one carbon. CH4 gives a "quintet", CH3Cl a "quartet", CH2Cl2 a "triplet" and CHCl3 a "doublet".
Doesn't it remind you of the "N+1" rule? Which atom is responsible of N? Yes, right, hydrogen.
The 1.1% of the molecules containing 13C do contain 100% of 1H and 13C is thus "influenced" by the 1H tiny magnets.
Magnets are magnets and they influence one another regardless whether they belong to the same type of nuclei or not.
Keep this in mind:
13C-NMR spectra are always recorded "1H-uncoupled".
In that way spectra result very simple and sensitivity is gained because every multiplet is made coalesce to a single signal.
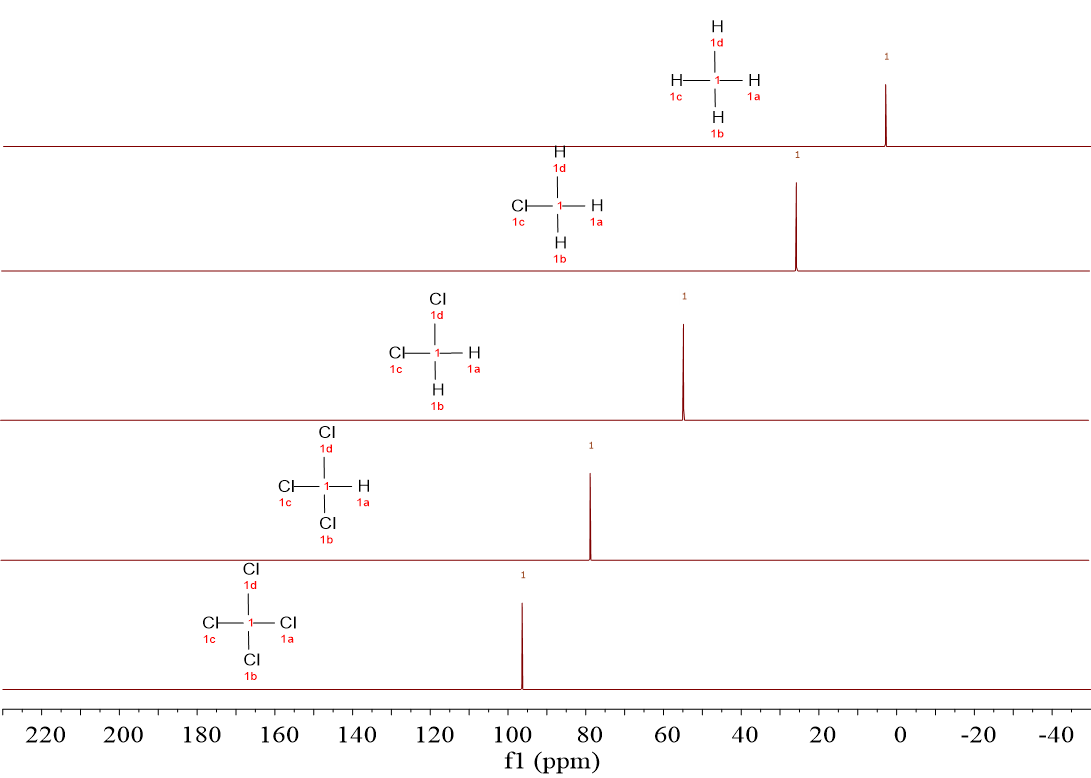
Look to this series. The five 13C spectra are 1H-decoupled. CCl4 has been also included for the sake of comparison.
The first feature gleaming to your eyes should be the horizontal scale that, instead of being in the tens of ppm, it is in the hundreds of ppm.
Which are the 13C chemical shifts of these molecules?
From 0.0 ppm in CH4 to almost 100 ppm in CCl4, passing through progressive intermediate situations with the increasing number of Cl atoms.
Does it make any sense?
Of course it does: The larger the number of Cl atoms, the higher the chemical shift. It's exactly the same as in 1H-NMR, isn't it?
You just re-discovered the first criterium to 13C-NMR spectra deciphering:
The 13C chemical shift depends on the 13C chemical environment, i.e. on the nature of its neighboring atoms.
Let's look at two additional spectra, those corresponding to ClCH2CH2CH3 and CH3CHClCH3 that we already analyzed in 1H-NMR.
Anything calling your atention? Several things for sure.
But before keeping on reading, make a short list and then check whether we agree.
1) Each spectrum contains more than one signal.
2) No integral was measured.
3) Chemical shifts are different.
4) All signals are singlets.
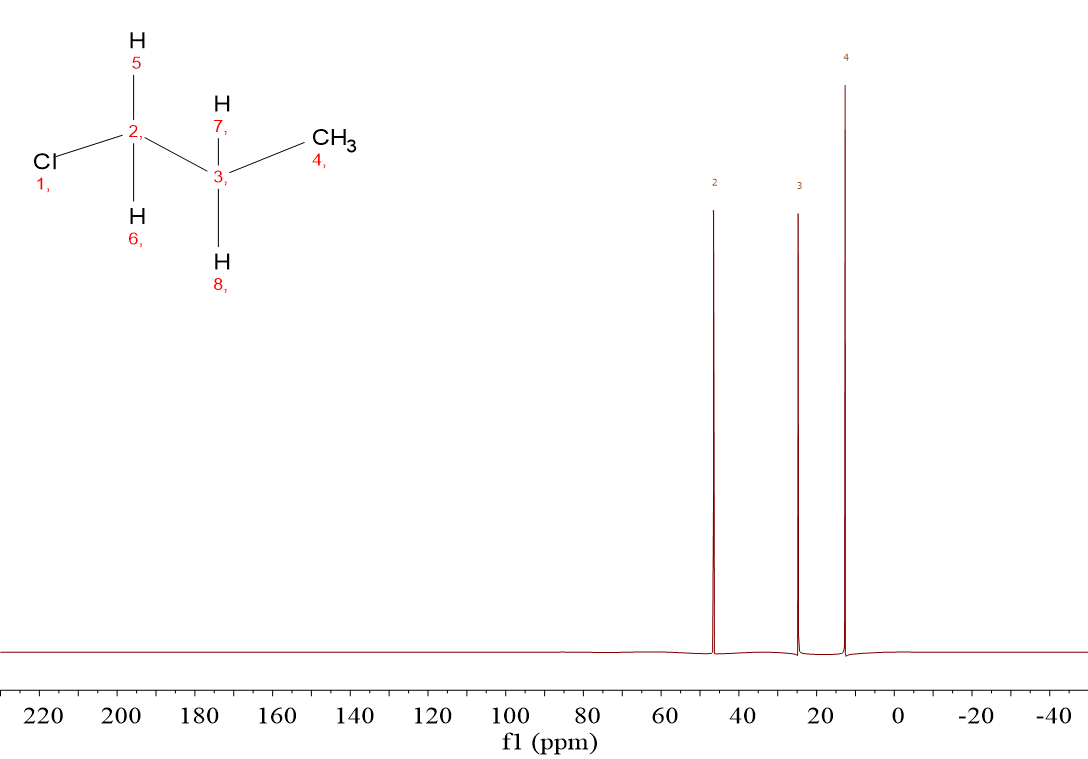 1-Chloropropane (ClCH2CH2CH3) gives rise to three signals because it contains three kinds of 13C: that of the ClCH2 group, that of the CH2 group in the middle of the molecule and that of the CH3 group.
1-Chloropropane (ClCH2CH2CH3) gives rise to three signals because it contains three kinds of 13C: that of the ClCH2 group, that of the CH2 group in the middle of the molecule and that of the CH3 group.
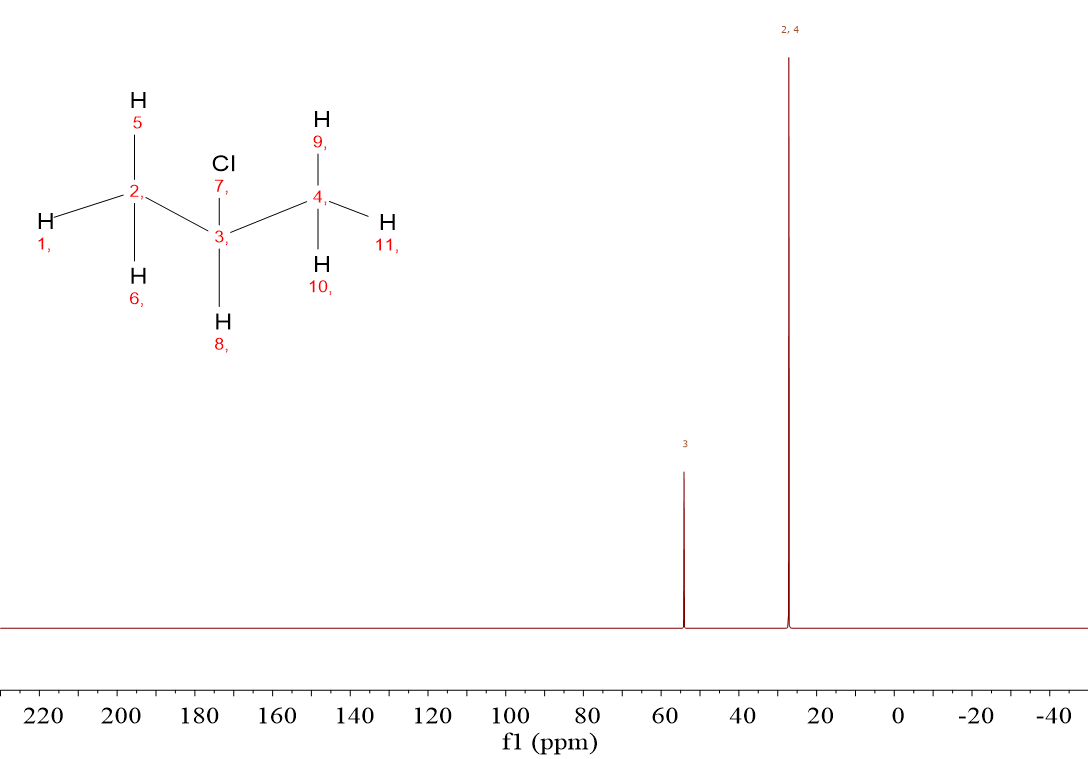 2-Chloropropane (CH3CHClCH3) displays two signals because there are only two types of 13C: that of the CHCl group in one hand and those of the two equivalent CH3 groups on the other.
2-Chloropropane (CH3CHClCH3) displays two signals because there are only two types of 13C: that of the CHCl group in one hand and those of the two equivalent CH3 groups on the other.
 The three signals belonging to ClCH2CH2CH3 have no measured integral because in 13C-NMR, intensity does not exactly correspond to the number of carbons. Besides, 13C signals seldom belong to more than one carbon each.
The three signals belonging to ClCH2CH2CH3 have no measured integral because in 13C-NMR, intensity does not exactly correspond to the number of carbons. Besides, 13C signals seldom belong to more than one carbon each.
 The two signals of CH3CHClCH3 have no integral because in 13C-NMR, intensity does not exactly correspond to the number of carbons.
The two signals of CH3CHClCH3 have no integral because in 13C-NMR, intensity does not exactly correspond to the number of carbons.
In this case the CH3 groups are equivalent and give a more intense signal that nevertheless is not exactly double than the other.
 For ClCH2CH2CH3, chemical shifts are 45 ppm, 25 ppm and 12 ppm.
For ClCH2CH2CH3, chemical shifts are 45 ppm, 25 ppm and 12 ppm.
How does one assign the 13C signals?
Your guess is right: by the 13C proximity to Cl.
One must assign the signal at the highest chemical shift (45 ppm) to the CH2Cl group whilst that at 25 ppm must be awarded to the CH2 group in the middle of the molecule.
The closer the proximity of 13C to Cl, measured in number of bonds, the higher their chemical shift:
CH2Cl (1 bond), 25 ppm
CH2 in the middle (2 bonds), 25 ppm and CH3 (3 bonds), 12 ppm.
 For CH3CHClCH3 it is observed a very high chemical shift (52 ppm) and another lower one (25 ppm).
For CH3CHClCH3 it is observed a very high chemical shift (52 ppm) and another lower one (25 ppm).
Do the chemical shifts make sense?
Yes indeed, they do!!!
The 13C of CH is at 1 bond from Cl whereas 13C's of CH3 are at 2 bonds from Cl.
The closer to the electronegative atom, the higher the chemical shift.
As it has earlier been pointed out, all signals are singlets.
If they weren't singlets and 13C were thus allowed to show their multiplicity coming from their attached 1H's, spectra would be a terrible tangle of signals.
We lose however important information as to how many 1H's the 13C'sare bonded to.
There are other methods to gather that info, still keeping the siganals be singlets. We'll see this in the following sections.


 1-Chloropropane (ClCH2CH2CH3) gives rise to three signals because it contains three kinds of 13C: that of the ClCH2 group, that of the CH2 group in the middle of the molecule and that of the CH3 group.
1-Chloropropane (ClCH2CH2CH3) gives rise to three signals because it contains three kinds of 13C: that of the ClCH2 group, that of the CH2 group in the middle of the molecule and that of the CH3 group.
 2-Chloropropane (CH3CHClCH3) displays two signals because there are only two types of 13C: that of the CHCl group in one hand and those of the two equivalent CH3 groups on the other.
2-Chloropropane (CH3CHClCH3) displays two signals because there are only two types of 13C: that of the CHCl group in one hand and those of the two equivalent CH3 groups on the other.
 The three signals belonging to ClCH2CH2CH3 have no measured integral because in 13C-NMR, intensity does not exactly correspond to the number of carbons. Besides, 13C signals seldom belong to more than one carbon each.
The three signals belonging to ClCH2CH2CH3 have no measured integral because in 13C-NMR, intensity does not exactly correspond to the number of carbons. Besides, 13C signals seldom belong to more than one carbon each.
 The two signals of CH3CHClCH3 have no integral because in 13C-NMR, intensity does not exactly correspond to the number of carbons.
The two signals of CH3CHClCH3 have no integral because in 13C-NMR, intensity does not exactly correspond to the number of carbons. For ClCH2CH2CH3, chemical shifts are 45 ppm, 25 ppm and 12 ppm.
For ClCH2CH2CH3, chemical shifts are 45 ppm, 25 ppm and 12 ppm. For CH3CHClCH3 it is observed a very high chemical shift (52 ppm) and another lower one (25 ppm).
For CH3CHClCH3 it is observed a very high chemical shift (52 ppm) and another lower one (25 ppm).
