Let's start deciphering spectra...
Look at these four 1H-NMR spectra. They belong to four very simple alike molecules, yet the spectra are rather different:
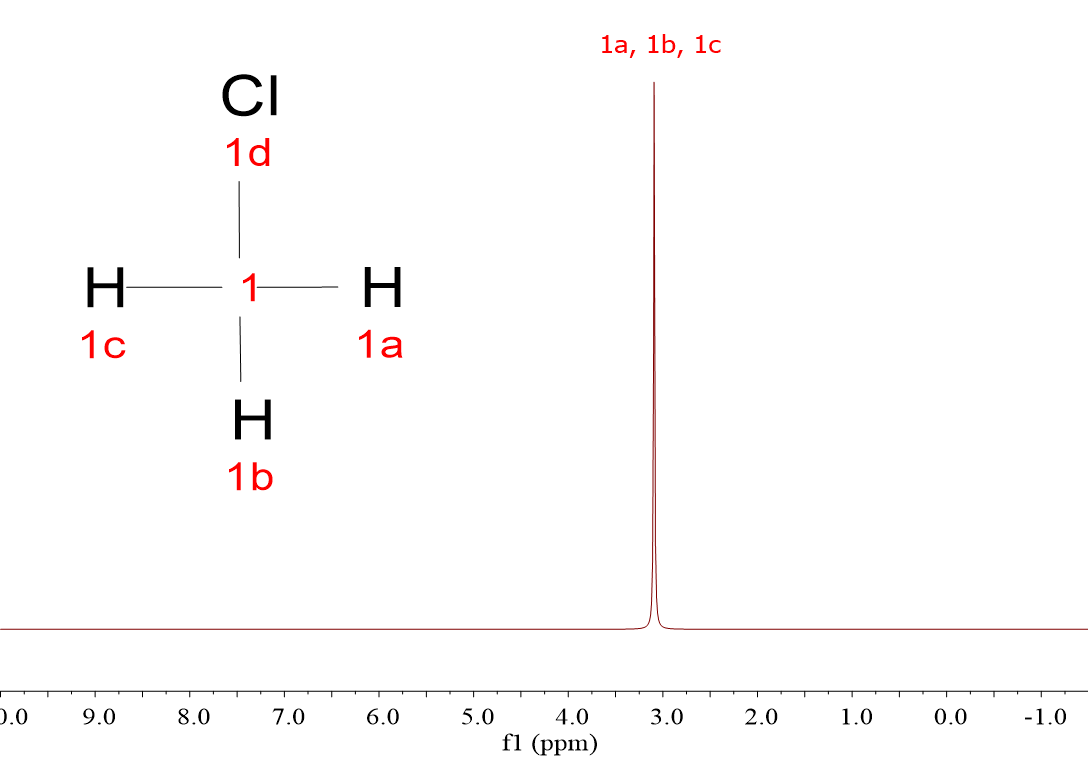
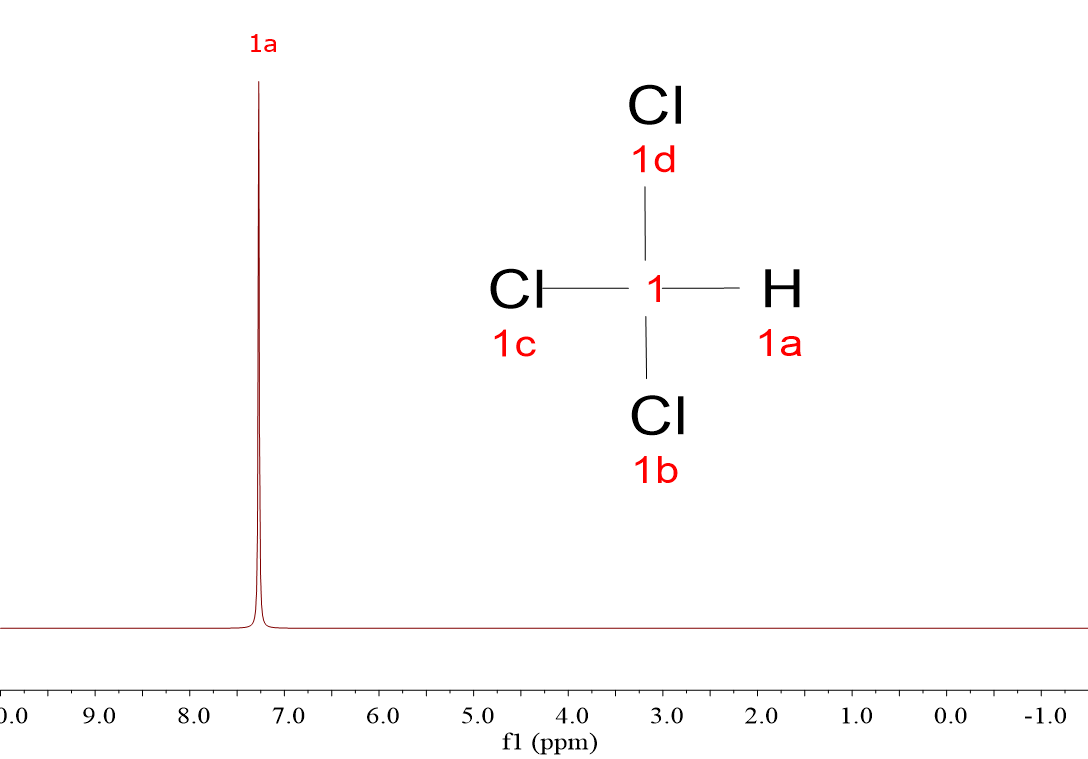
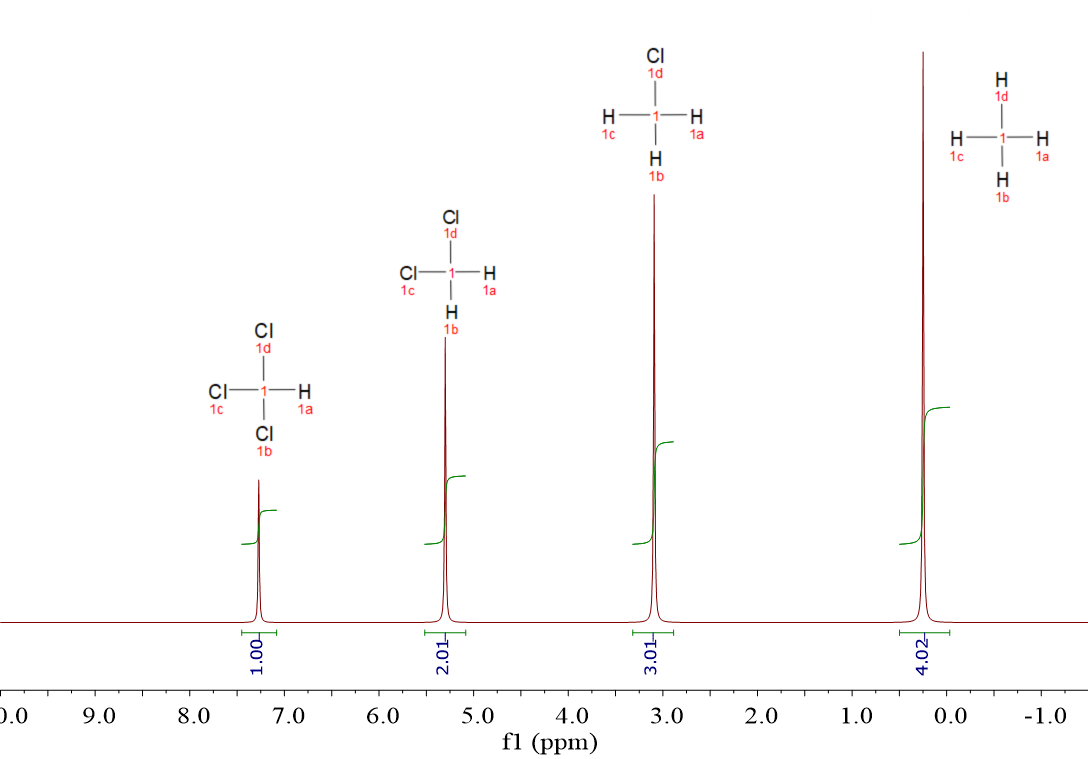
CH4, CH3Cl, CH2Cl2 and CHCl3.
What do you see?
The four spectra display one single signal, even though the majority of the molecules bear more than one 1H each. The 1H within a molecule are equivalent and resonate together.
Did you notice where the signals showed up in the horizontal scale?
Yes, right, the more Cl atoms, the more shifted the signal to the left, towards higher "chemical shifts".
You just uncovered the first criterium to 1H-NMR spectra deciphering:
1H's resonate at different chemical shift depending on their chemical environment, i.e. their sorrounding atoms or groups.
Now, please, assume that we mix equimolecular amounts of each of the four molecules and record the 1H-NMR spectrum to the mixture.
Can you bet what's going to happen?
You bet! Four signals at the individual compound's chemical shifts.
Yet, did you notice something else?
Of course you did: the intensities where not equal, even though we made the number of molecules be exactly the same.
Why was that?
Because the molecules bear a different number of 1H. We meausured the signal's integrals and note that the relative areas correspond to the number of 1H each molecule has.
You just discovered the second criterium to 1H-NMR spectra deciphering:
The area (integral) of each signal is proportional to the number of 1H's giving rise to the signal.
Let's look at two additional spectra, those corresponding to ClCH2CH2CH3 and CH3CHClCH3.
Anything calling your atention? Several things for sure.
But before keeping on reading, make a short list and then check whether we agree.
1) Each spectrum contains more than one signal.
2) Each spectrum shows its own integral pattern.
3) Chemical shifts are different.
4) The signals display shapes of their own.
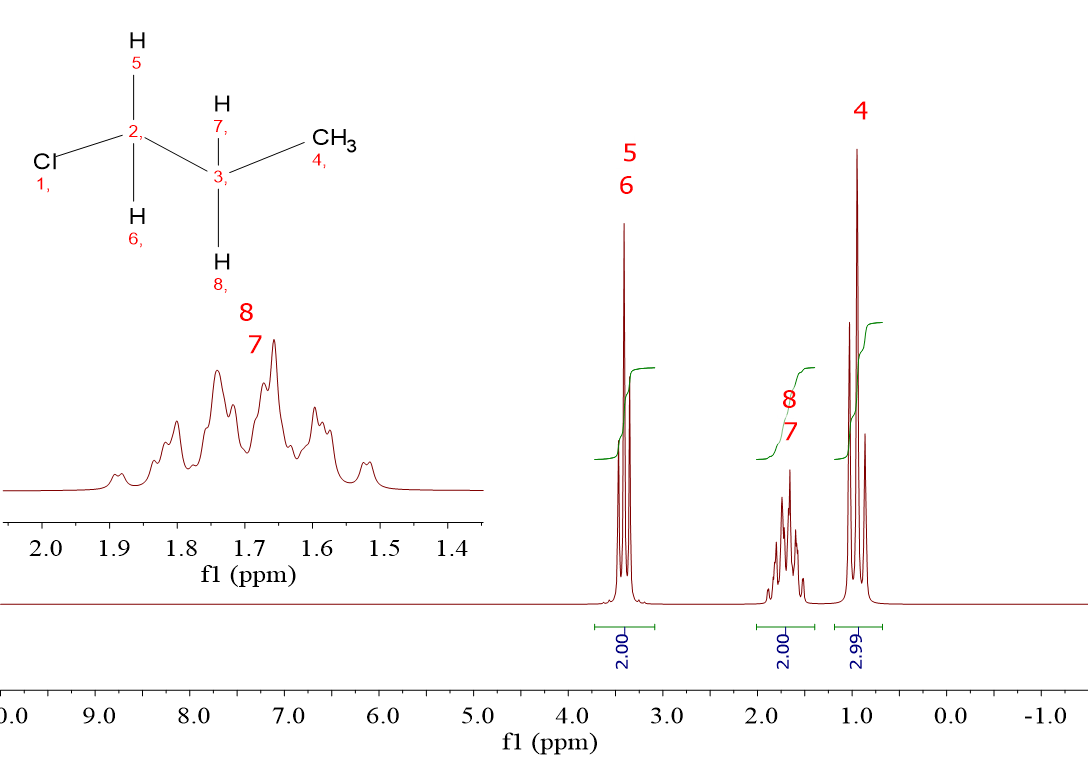 1-Chloropropane (ClCH2CH2CH3) gives rise to three signals because it contains three kinds of 1H: those of the ClCH2 group, those of the CH2 group in the middle of the molecule and those of the CH3 group.
1-Chloropropane (ClCH2CH2CH3) gives rise to three signals because it contains three kinds of 1H: those of the ClCH2 group, those of the CH2 group in the middle of the molecule and those of the CH3 group.
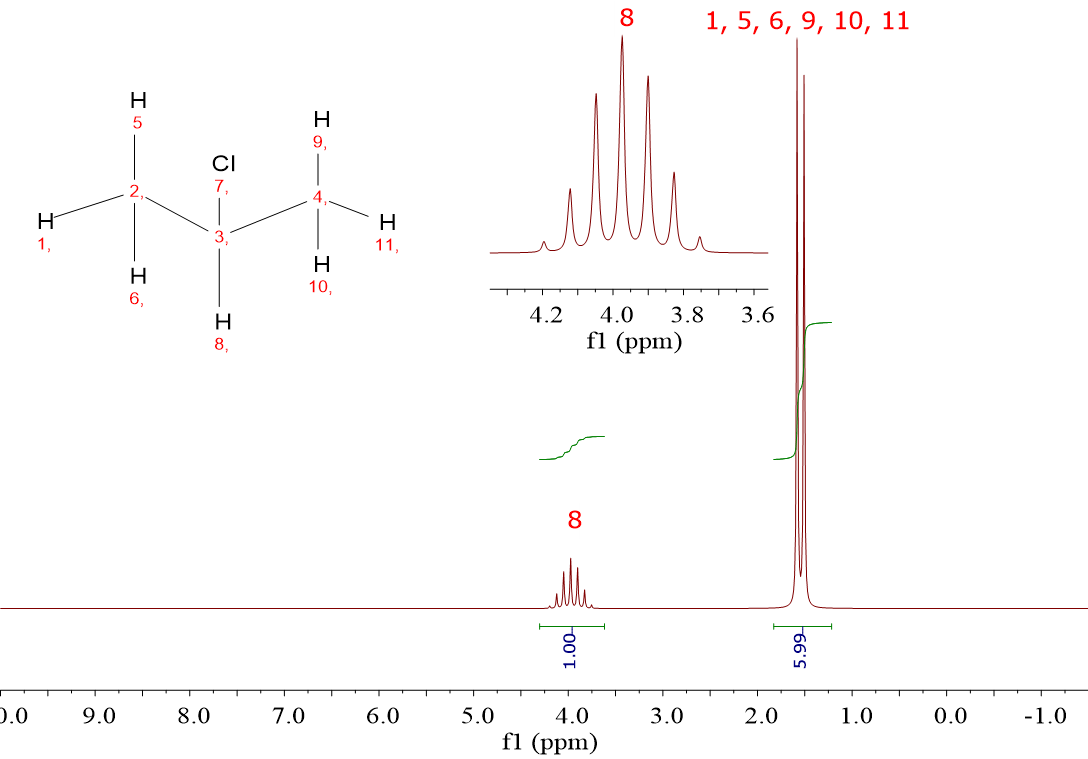 2-Chloropropane (CH3CHClCH3) displays two signals because there are only two types of 1H: that of the CHCl group in one hand and those of the two equivalent CH3 groups on the other.
2-Chloropropane (CH3CHClCH3) displays two signals because there are only two types of 1H: that of the CHCl group in one hand and those of the two equivalent CH3 groups on the other.
 The three signals belonging to ClCH2CH2CH3 bear a set of 2:2:3 integrals, in accordance to the number of 1H in each group.
The three signals belonging to ClCH2CH2CH3 bear a set of 2:2:3 integrals, in accordance to the number of 1H in each group.
 The two signals of CH3CHClCH3 display a 1:6 integral set, also in agreement with the number of 1H withing each group.
The two signals of CH3CHClCH3 display a 1:6 integral set, also in agreement with the number of 1H withing each group.
 For ClCH2CH2CH3, chemical shifts are 3.4 ppm, 1.7 ppm and 0.9 ppm, the latter signal bearing an integral of 3 and thus no doubt belonging to the CH3.
For ClCH2CH2CH3, chemical shifts are 3.4 ppm, 1.7 ppm and 0.9 ppm, the latter signal bearing an integral of 3 and thus no doubt belonging to the CH3.
How does one assign the other two signals if their integrals are the same?
Your guess is right: by the group's proximity to Cl.
One must assign the signal at the highest chemical shift (3.4 ppm) to the CH2Cl group whilst that at 1.7 ppm must be awarded to the CH2 group in the middle of the molecule.
The closer the proximity of 1H's to Cl, measured in number of bonds, the higher their chemical shift:
CH2Cl (2 bonds), 3.4 ppm
CH2 in the middle (3 bonds), 1.7 ppm and CH3 (4 bonds), 0.9 ppm.
 For CH3CHClCH3 it is observed a very high chemical shift (3.95 ppm) and another much lower one (1.5 ppm).
For CH3CHClCH3 it is observed a very high chemical shift (3.95 ppm) and another much lower one (1.5 ppm).
The integrals leave us with no doubt concerning their assignment:
CH, one 1H, 3.95 ppm
(CH3)2, six 1H, 1.5 ppm
Do the chemical shifts make sense?
Yes indeed, they do!!!
The 1H of CH is at 2 bonds from Cl whereas 1H'sof CH3 are at 3 bonds from Cl.
The closer to the electronegative atom, the higher the chemical shift.
 Last but not least, please look at the signals' shape:
Last but not least, please look at the signals' shape:
For ClCH2CH2CH3 there's a “triplet” at 0.9 ppm, a “complex sextet” at 1.7 ppm and another “triplet” at 3.4 ppm.
The signal at 0.9 ppm belonging to the CH3 group is a "triplet" because its vicinal group, the CH2 in the middle of the molecule, has two 1H's.
The rule of simple coupling says that the multiplicity of a signal equals the number of vicinal 1H's plus one (N+1).
THE VICINALHOOD IS REDUCED JUST TO THE CONTIGUOUS POSITION OF THE CONSIDERED 1H's.
That's why the ClCH2 did show up as a "triplet”, due to the two vicinal 1H's of the CH2 group in the middle.
How many neighboring 1H's does the CH2 in the middle have?
It's got 5, hasn't it? Two from the CH2Cl group and three from the CH3 group.
Following the N+1 rule it's got to give a sextet, as it really does.
However, the fact that the CH2Cl and CH3 groups are non-equivalent makes the sextet be certainly complex. This is a story to be told further on.
 Last but not least, please look at the signals' shape:
Last but not least, please look at the signals' shape:
For CH3CHClCH3 there's a “septet” at 3.95 ppm and a “doublet” at 1.5 ppm.
The six equivalent 1H's of the two equivalent CH3 groups have only one neighbor: the 1H of the CH.
The N+1 rule applies and a "doublet” is obtained.
An easy question: Why the signal belonging to the CH group shows up as a "septet"?
Piece of cake!!!
Because its number of vicinal 1H's is six from the two CH3 groups, isn't it?
Just apply the N+1 rule and you got it!!!
You just discovered a third criterium to 1H-NMR spectra deciphering: the "proton couplings".
The vicinal protons couple one another and their coupling produces signal splitting that informs us about the number of neighboring 1H's each group bears.




 1-Chloropropane (ClCH2CH2CH3) gives rise to three signals because it contains three kinds of 1H: those of the ClCH2 group, those of the CH2 group in the middle of the molecule and those of the CH3 group.
1-Chloropropane (ClCH2CH2CH3) gives rise to three signals because it contains three kinds of 1H: those of the ClCH2 group, those of the CH2 group in the middle of the molecule and those of the CH3 group.
 2-Chloropropane (CH3CHClCH3) displays two signals because there are only two types of 1H: that of the CHCl group in one hand and those of the two equivalent CH3 groups on the other.
2-Chloropropane (CH3CHClCH3) displays two signals because there are only two types of 1H: that of the CHCl group in one hand and those of the two equivalent CH3 groups on the other.
 The three signals belonging to ClCH2CH2CH3 bear a set of 2:2:3 integrals, in accordance to the number of 1H in each group.
The three signals belonging to ClCH2CH2CH3 bear a set of 2:2:3 integrals, in accordance to the number of 1H in each group.
 The two signals of CH3CHClCH3 display a 1:6 integral set, also in agreement with the number of 1H withing each group.
The two signals of CH3CHClCH3 display a 1:6 integral set, also in agreement with the number of 1H withing each group.
 For ClCH2CH2CH3, chemical shifts are 3.4 ppm, 1.7 ppm and 0.9 ppm, the latter signal bearing an integral of 3 and thus no doubt belonging to the CH3.
For ClCH2CH2CH3, chemical shifts are 3.4 ppm, 1.7 ppm and 0.9 ppm, the latter signal bearing an integral of 3 and thus no doubt belonging to the CH3. For CH3CHClCH3 it is observed a very high chemical shift (3.95 ppm) and another much lower one (1.5 ppm).
For CH3CHClCH3 it is observed a very high chemical shift (3.95 ppm) and another much lower one (1.5 ppm). Last but not least, please look at the signals' shape:
Last but not least, please look at the signals' shape: Last but not least, please look at the signals' shape:
Last but not least, please look at the signals' shape: