MY FIRST 13C-NMR SPECTRUM
Let me introduce you the 13C-NMR spectrum of ibuprofen.
You have surely taken it many times. This analgesic drug has the molecular formula C13H18O2.
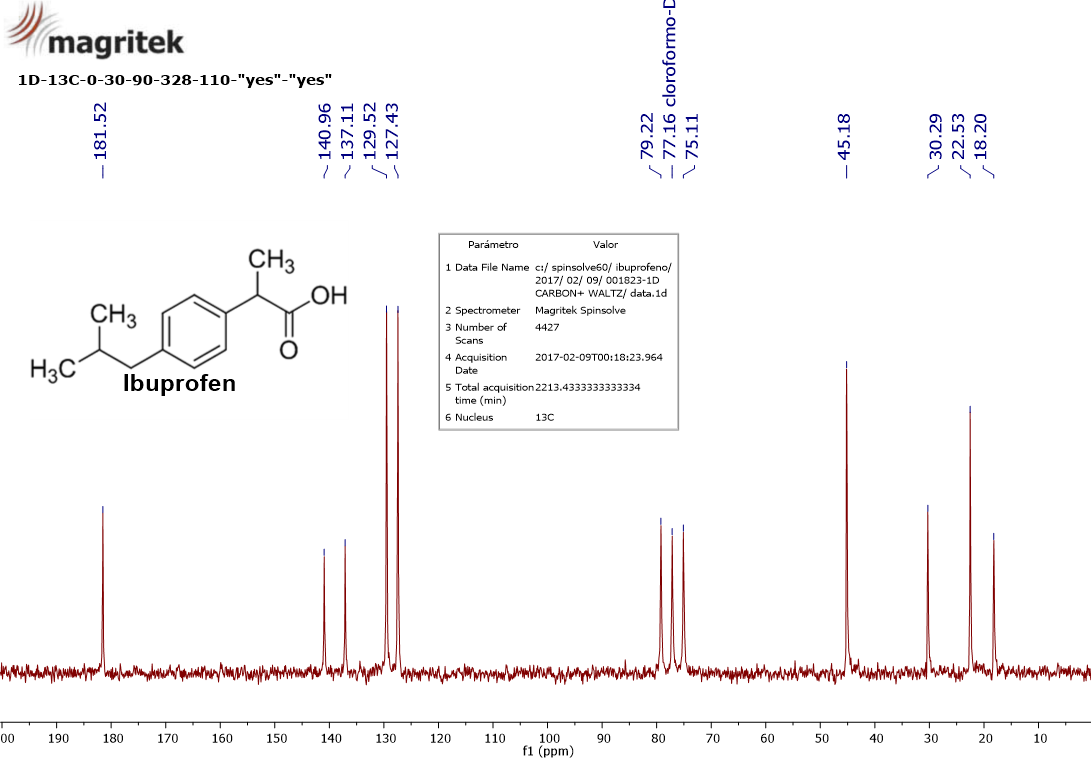 13C-NMR of ibuprofen.
13C-NMR of ibuprofen.
All NMR spectra, regardless of the nucleus one is observing, consist of an horizontal scale, the "chemical shift".
In 13C the scale spans from 0 to 250 ppm (parts per million; we'll explain the meaning of that very soon!!!), that compares with the usual 1H range of only 0 to 15 ppm.
The different carbons of the given structure show up on that scale at different "chemical shift" or position. Had it not been for that, NMR wouldn't have been of any use to solve structures.
Let's count and single out the different carbons of ibuprofen. To do that we'll use symmetry criteria and the fact that single bonds freely rotate like a pinwheel. Please, read mindfully the following, even though you might miss something yet.
One may wonder, are the three methyl groups equivalent?
There are two equivalent ones: those of the isopropyl group (CH3)2C. Their imaginary exchange leaves ibuprofen untouched. We thus expect these two methyl carbons to share the same "address" or "chemical shift".
The other methyl group is at a very different location and therefore differs in "address" to the other two.
Summing up, we expect the methyl groups appearing at two different "chemical shifts", one for the two carbons of the isopropyl group and another for the other methyl group.
There is an unique CH2 in the molecule. Here we have another "address".
We discover two kinds of CH groups: four belonging to the ring and two outside the ring. The latter are non-symmetry related and their carbons are expected to dwell in two different "addresses". Those belonging to the ring are symmetry related by pairs but each pair looks quite alike to one another. Consequently we should expect two "addresses" very close by in the neighborhood.
Last but not least, the "non-hydrogenated" carbons have still to be accounted for. There are two of them in the ring and that of the unique COOH.
How many "addresses" did we single out?
1) That of the equivalent (CH3)2 groups with two carbons.
2) That of the other methyl group.
3) That of the sole CH2.
4) Those of the CH groups outside the ring.
5) Those of each pair of CH groups inside the ring, each one with two carbons.
6) Those of the two "non-hydrogenated" carbons in the ring.
7) That of the COOH group.
We singled out ten distinct "addresses", each one with one or two "dwellers".
Take a closer look to the spectrum and its horizontal scale. I want you to discover nine signals:
18.2 ppm
22.5 ppm
30.3 ppm
45.2 ppm
127.4 ppm
129.5 ppm
137.1 ppm
141.0 ppm
181.5 ppm
Agreed?
 13C-NMR of ibuprofen.
13C-NMR of ibuprofen.
It should be obvious to the naked eye the simplicity of the 13C spectrum as compared to 1H.
In the 13C-NMR spectra the signals are all "singlets" with the exception of the solvent that one must learn how to discern. In this case the solvent is deuterated chloroform (CDCl3; we'll explain that further on) which gives the “triplet” centered at 77.2 ppm.
Another outstanding feature is the "chemical shift" scale that extends to 250 ppm, instead of the 15 ppm in 1H.
Another important peculiarity is the ABSENCE OF INTEGRAL.
Unfortunately, in the case of 13C, signal areas don't go with the number of carbons as they reliably did in 1H.
Let's interpret as much as we can:
How many carbons does ibuprofen have?
In its formula C13H18O2 there are 13. Do we have 13 13C signals? We only observe 9, apart from the solvent's three.
Remember that there were two equivalent methyl groups. Their carbons will be equivalent as well. Taking that into account we should expect twelve signals, still too many.
And the ring? There are two pairs of equivalent carbons, the upper CH and the lower CH. Deducing those equivalencies, we still should expect ten signals.
There should exist an additional equivalency not explained by symmetry but by mere accident.
That certainly is a difficulty of NMR: accidental coincidence of signals.
We'll deal with that in the next sections.
Talking about equivalencies and accidental coincidences, look now at the spectrum and note that there are four signals with an average intensity kind of double that the average rest.
That usually is an indication of equivalency or coincidence of "chemical shift" of two carbons.
If we consider the signals at 22.5 ppm, 45.2 ppm, 127,4 ppm and 129.5 ppm as approximately “double” in intensity, we'll have explained the 13 carbons of ibuprofen's molecular formula.
Believe it or not, you already understood your first 13C-NMR spectrum.
Please, don't fret!!!. This is your first approach to spectra interpretation. And you know almost nothing as yet !!!
Step by step…
 13C-NMR of ibuprofen.
13C-NMR of ibuprofen.
 13C-NMR of ibuprofen.
13C-NMR of ibuprofen.
