Let me introduce you the 1H-NMR spectrum
of ibuprofen.
You have surely taken it many times. This analgesic drug has the molecular formula C13H18O2.
 1H-NMR spectrum of ibuprofen.
1H-NMR spectrum of ibuprofen.
1H-NMR spectra consist of an horizontal scale, the "chemical shift", usually from 0 to 15 ppm (parts per million; we'll explain the meaning of that very soon!!!).
The different hidrogens of the given structure show up on that scale at different "chemical shift" or position.
Had it not been for that, NMR wouldn't have been of any use to solve structures.
Let's count and single out the different hydrogens of ibuprofen.
To do that we'll use symmetry criteria and the fact that single bonds freely rotate like a pinwheel.
Please, read mindfully the following, even though you might miss something yet.
In each of the methyl groups (CH3), the three hydrogens bear equivalent positions because of the C-C bond rotation, i.e. they are exchangeable without affecting the molecular integrity. That's why they are expected to give a single signal.
It's like the CH3 hydrogens share the same "address" or "chemical shift".
One may wonder, are the three methyl groups equivalent?
There are two equivalent ones: those of the isopropyl group (CH3)2C. Their imaginary exchange leaves ibuprofen untouched. We thus expect these six hydrogens to share the same "address" or "chemical shift".
The other methyl group is at a very different location and therefore differs in "address" to the other two.
Summing up, we expect the methyl groups appearing at two different "chemical shifts", one for the six hydrogens of the isopropyl group and another for the other methyl group.
There is a unique CH2 in the molecule whose hydrogens are equivalent to each other. Here we have another "address".
We discover two kinds of CH groups: four belonging to the ring and two outside the ring. The latter are non-symmetry related and are expected to dwell in two different "addresses".
Those belonging to the ring are symmetry related by pairs but each pair looks quite alike to one another. Consequently we should expect two "addresses" very close by in the neighborhood.
Last but not least, the OH's hydrogen is clearly unique.
How many "addresses" did we single out?
1) That of the equivalent (CH3)2 groups mooring six hydrogens to it.
2) That of the other methyl group with three hydrogens.
3) That of the sole CH2.
4) Those of the CH groups outside the ring, with one hydrogen each.
5) Those of each pair of CH groups inside the ring, each one with two hydrogens.
6) That of the OH group.
We singled out eight distinct "addresses", each one with a different number of "dwellers".
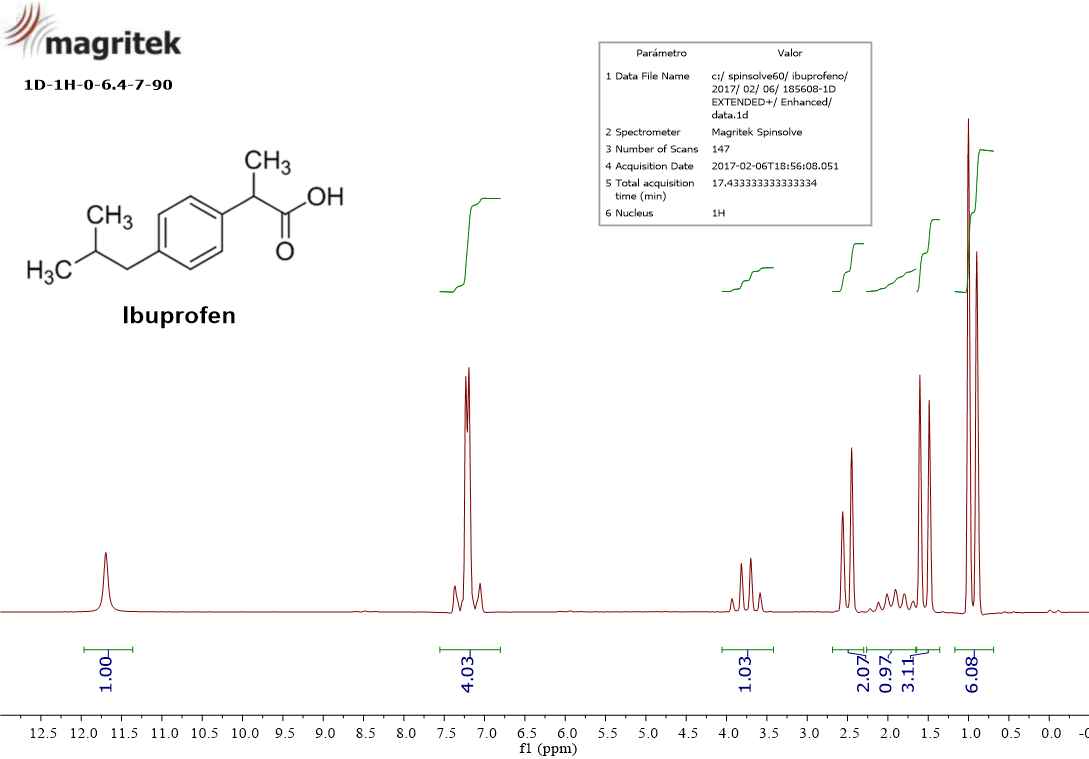
Take a closer look to the spectrum and its horizontal scale.
I want you to discover seven signals:
0.9 ppm
1.5 ppm
1.8 ppm
2.5 ppm
3.7 ppm
7.2 ppm
11.7 ppm
Agreed?
 1H-NMR spectrum of ibuprofen.
1H-NMR spectrum of ibuprofen.
Please, notice that there is a green trace for each signal ("integral") that calculates the area enclosed by each signal, whose value is written just below the spectrum drawing.
Let's look at the spectrum again:
At 11.7 ppm there is a simple signal (“singlet”) whose “integral” or area is 1.0.
At 7.2 ppm there is a complex signal ("multiplet") whose area amounts 4.
At 3.7 ppm there is a complex signal (“quartet”), whose area is 1.0.
At 2.5 ppm there is a “doublet” whose integral or area is 2.
At 1.8 ppm there is a "multiplet" with an area of 1.0.
At 1.5 ppm there is a "doublet" whose area is 3.
At 0.9 ppm there finally is another "doublet" with an area of 6.
Does all this ring a bell?
Each signal is an address at a given "chemical shift" in the horizontal scale (ppm).
Each address has a different number of dwellers that means a distinct area or integral.
With that idea in mind, do you dear assigning signals to the different ibuprofen's hydrogens?
I'm sure you do, at least some of them...
Don't you remember that we expected a single address for the two equivalent methyls (CH3)2 with 6 hydrogens? Is there any signal displaying an area of 6? Right: the doublet at the chemical shift of 0.9 ppm.
There's another doublet at 1.5 ppm with an integral of 3 that must be assigned to the other methyl's address (CH3).
The doublet at 2.5 ppm bears an integral of 2, hence we can assign this address to the CH2 group.
The CH groups outside the ring, each one with one hydrogen, were very different addresses. Are there signals with unity areas? Those of 1.8 ppm and 3.7 ppm.
We can stand out the "odd" signal at 7.2 pmm with an integral of 4. Didn't we expect the in-the-ring CH groups to dwell in two close addresses? Here you are.
Wrapping up, the strange creature, the OH group, is left at 11.7 ppm.
Congrats!!! You mastered your first 1H-NMR spectrum!!!
The integrals were instrumental, weren't they?
The most important thing to be done when studying a 1H-NMR spectrum is to look at the integrals.
A careful integration is key to your assigning of signals to a structure.
We still have to understand why the signals show up at different "chemical shifts" and how come their looking as "singlets", "doublets", "triplets", "quartets", and more.
Let's go step by step...
 1H-NMR spectrum of ibuprofen.
1H-NMR spectrum of ibuprofen.
 1H-NMR spectrum of ibuprofen.
1H-NMR spectrum of ibuprofen.
