REVISION AND ADVANCED ASPECTS
I'd like to make you aware of the fact that NMR is quite complex a technique to master and its spectra are sometimes very intricate and difficult to interpret. In this course we are far from explaining everything but we'll provide you with enough experience in order to be way above the average person concerning structure determination.
Please, look carefully at the following spectra. They belong to some deuterated solvents that are currently used in NMR.
You have to learn how to discern those signals in your spectra.
There are many more solvents (benzene C6D6, acetone C3D6O, tetrahydrofuran C4H8O and a large etc.).
Peruse tables to know where they show up in the spectra.
 13C-NMR of CDCl3
13C-NMR of CDCl3
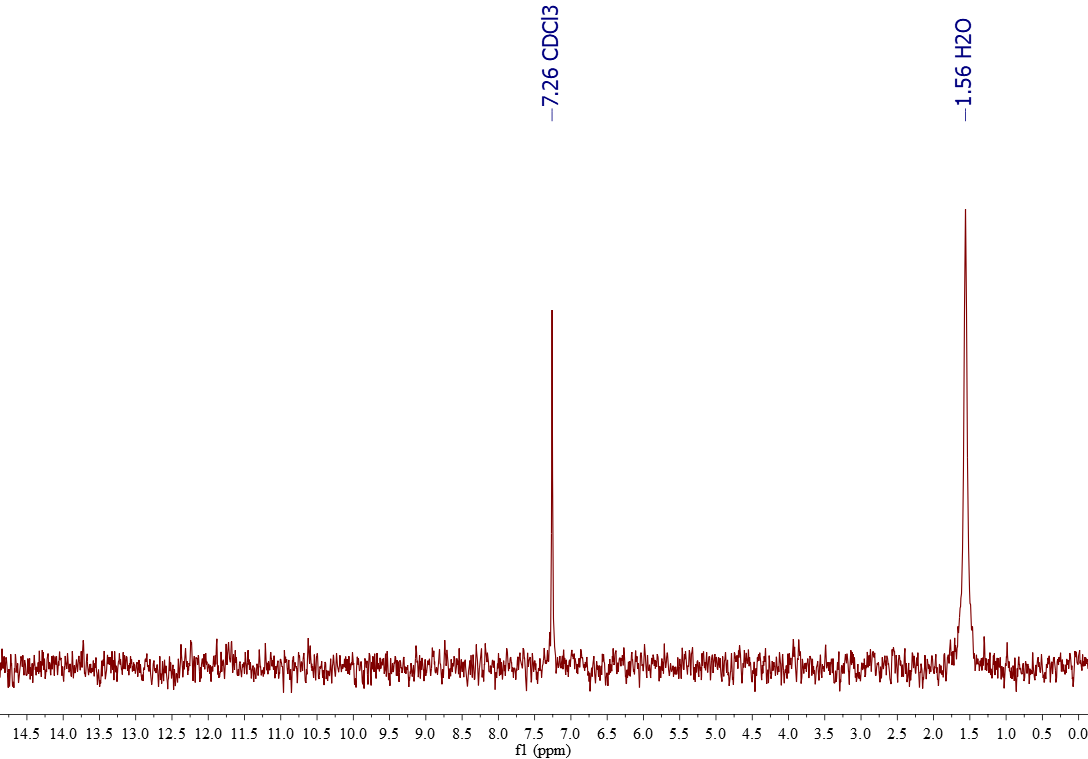 1H-NMR of CDCl3
1H-NMR of CDCl3
CDCl3 is usually sold at least 99% deuterium (D) rich. D is NMR-active and bear a magnetic moment I = 1.
Applying the rule "2NI+1" for the multiplicity, one gets
2·1·1+1 = 3.
That's why CDCl3 gives a triplet centered at 77.0 ppm.
However, since D has I = 1, the only D can adopt three arrangements in the magnetic field, instead of the two of nuclei with I = 1/2.
That makes too the 13C triplet intensity be 1:1:1.
The signals belong in true to the residual CHCl3 (1% or less; 7.26 ppm) and the tiny amount of water (ca. 1.5 ppm) that dissolves in CDCl3.
Water and chloroform are immiscible but a tiny amount of water always shows up in 1H-RMN.
One ought to reduce the intensity of the water signal by submiting CDCl3 to a drying process with a desiccant (CaCl2, molecular sieves, etc).
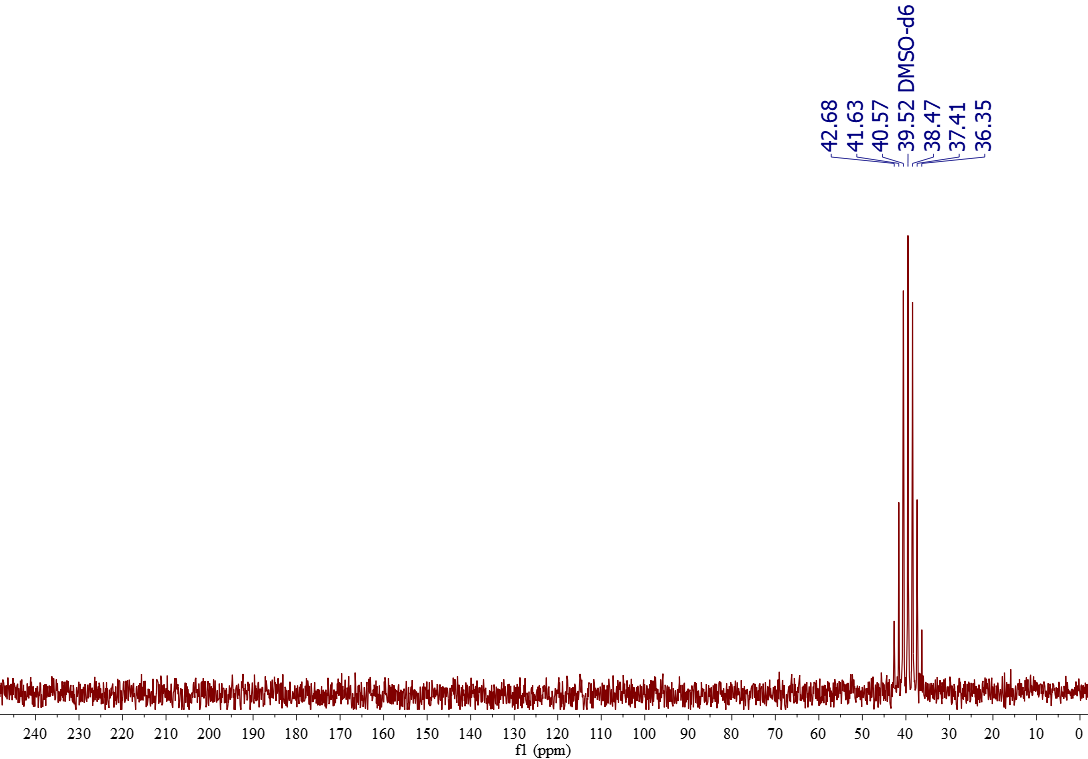 13C-NMR of DMSO-d6
13C-NMR of DMSO-d6
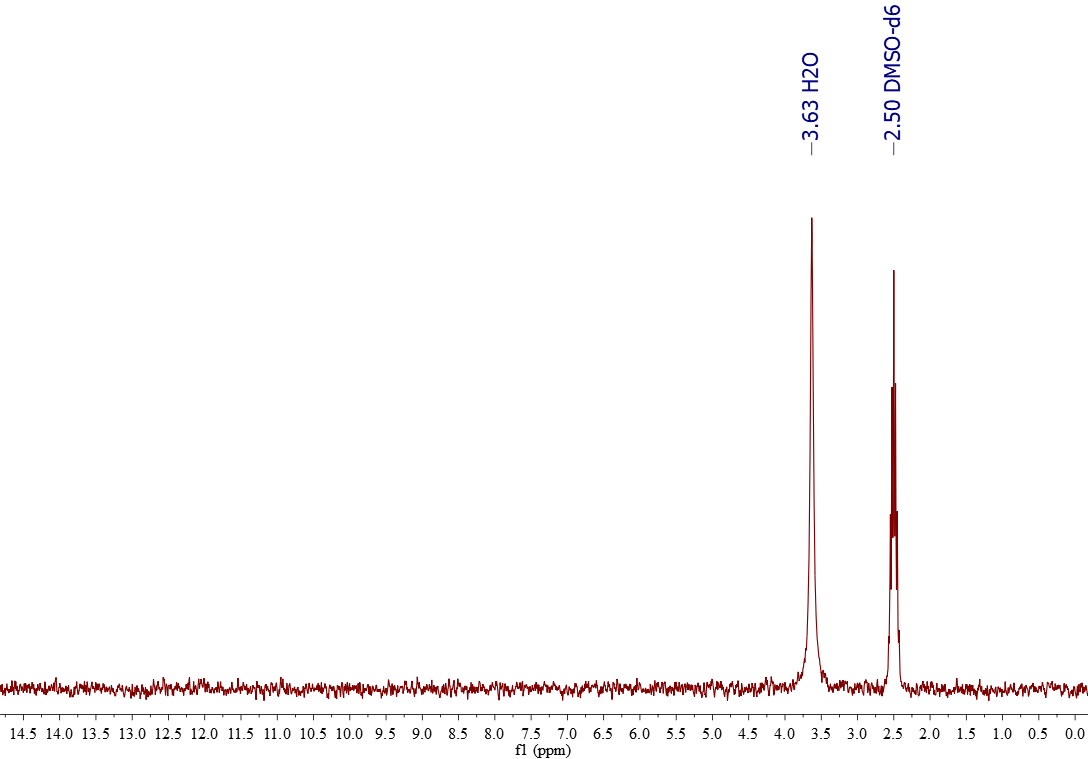 1H-NMR of DMSO-d6
1H-NMR of DMSO-d6
Deuterated dimethysulfoxide (DMSO-d6) is usually sold at least 99% deuterium (D) rich. D is NMR-active and bear a magnetic moment I = 1. Applying the rule "2NI+1" for the multiplicity, one gets 2·3·1+1 = 7 (the CD3 groups are equivalent). That's why DMSO-d6 gives a septet centered at 39.52 ppm.
The signals actually belong to the residual hidrogenated DMSO (1% or less; 2.50 ppm) and the amount of water (ca. 3.6 ppm) that dissolves in DMSO. Water and DMSO are miscible in any ratio. One ought to reduce the intensity of the water signal by drying DMSO-d6 with a non-basic desiccant (CaCl2, molecular sieves, etc).
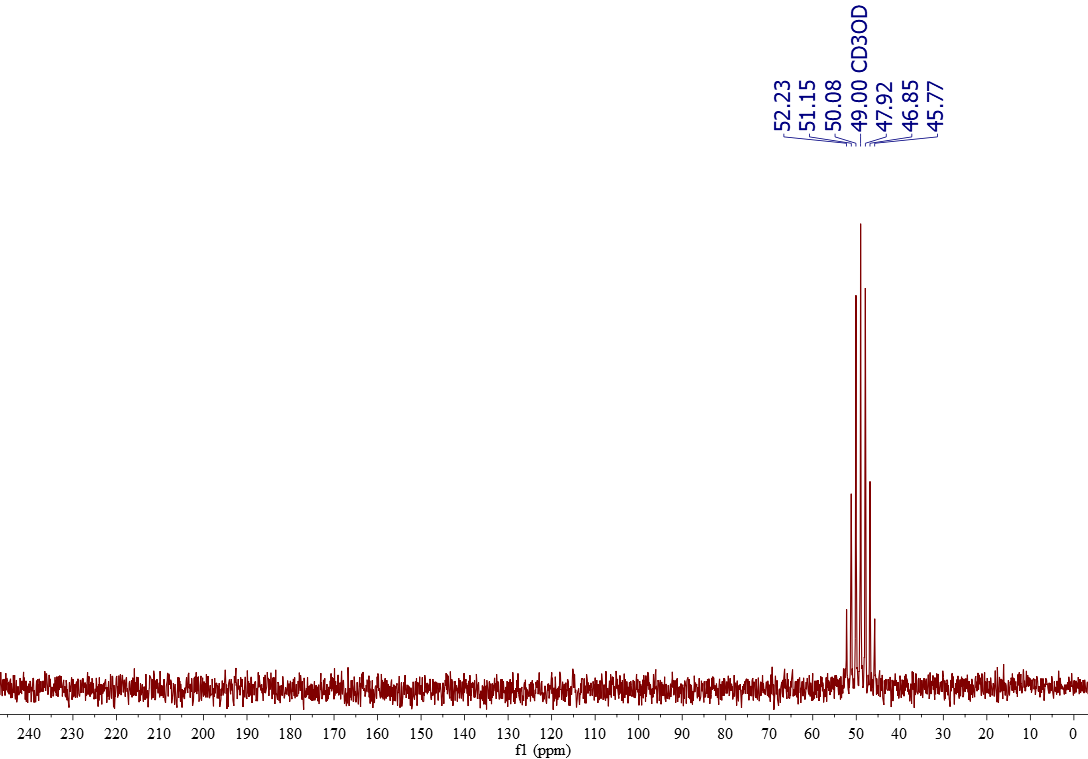 13C-NMR of CD3OD
13C-NMR of CD3OD
 1H-NMR of CD3OD
1H-NMR of CD3OD
Deuterated methanol (CD3OD) is usually sold at least 99% deuterium (D) rich. D is NMR-active and bear a magnetic moment I = 1. Applying the rule "2NI+1" for the multiplicity, one gets 2·3·1+1 = 7 . That's why CD3OD gives a septet centered at 49.0 ppm.
The signals actually belong to the residual CH3OH (1% or less; 3.31 ppm) and the amount of water (ca. 4.8 ppm) that dissolves in CD3OD. Water and CD3OD are miscible in any ratio. One ought to reduce the intensity of the water signal by drying CD3OD with a non-basic desiccant (CaCl2, molecular sieves, etc).
Why does one have to use deuterated solvents in NMR?
The solvent is the major component of a NMR sample, isn't it?. Had not the solvent been deuterated, its signal would dominate the spectrum and would overshadow those of the dissolved compound.
Deuterated solvents are essential in 1H-NMR and not that important in 13C-NMR.






 13C-NMR of CDCl3
13C-NMR of CDCl3
 1H-NMR of CDCl3
1H-NMR of CDCl3
 13C-NMR of DMSO-d6
13C-NMR of DMSO-d6
 1H-NMR of DMSO-d6
1H-NMR of DMSO-d6
 13C-NMR of CD3OD
13C-NMR of CD3OD
 1H-NMR of CD3OD
1H-NMR of CD3OD