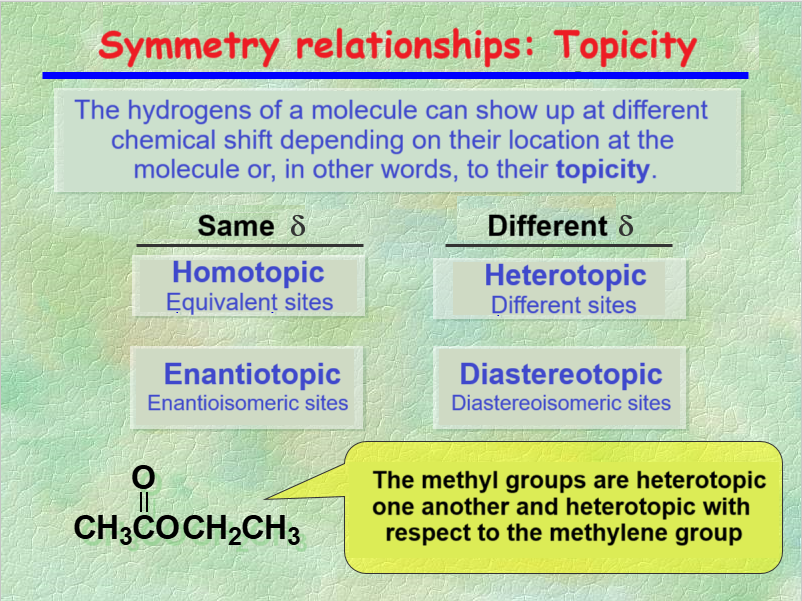
We've already seen that, to our fortune, the 1H's of a molecule give NMR signals at different chemical shifts, depending on their position within the molecule, i.e. their TOPICITY.
The nuclei groups in a molecule can have the indicated TOPICITIES.
Depending on their TOPICITY they'll show up at the same or different chemical shift.
Please, never forget that accidental coincidences do occur.
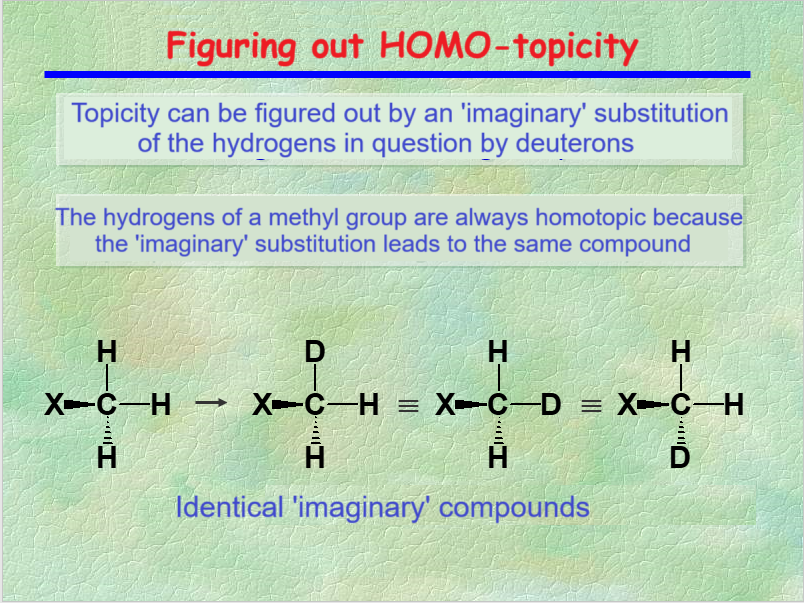
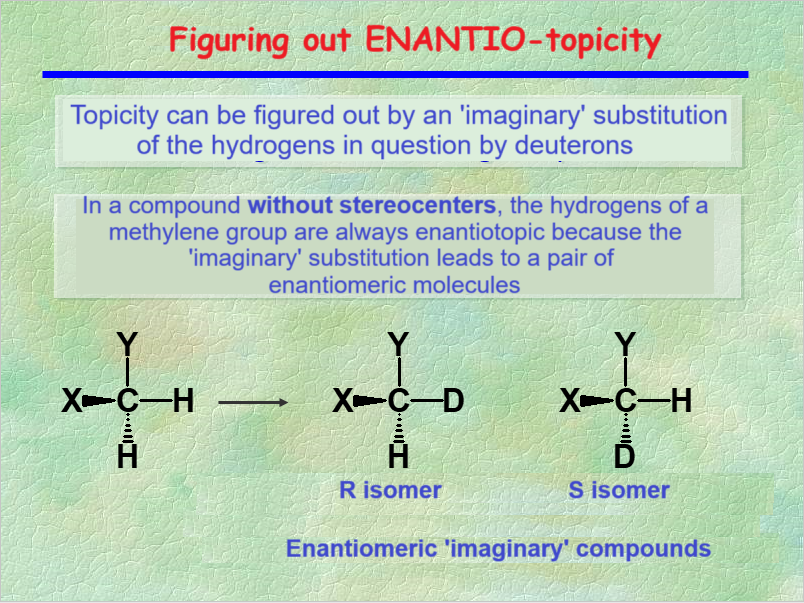
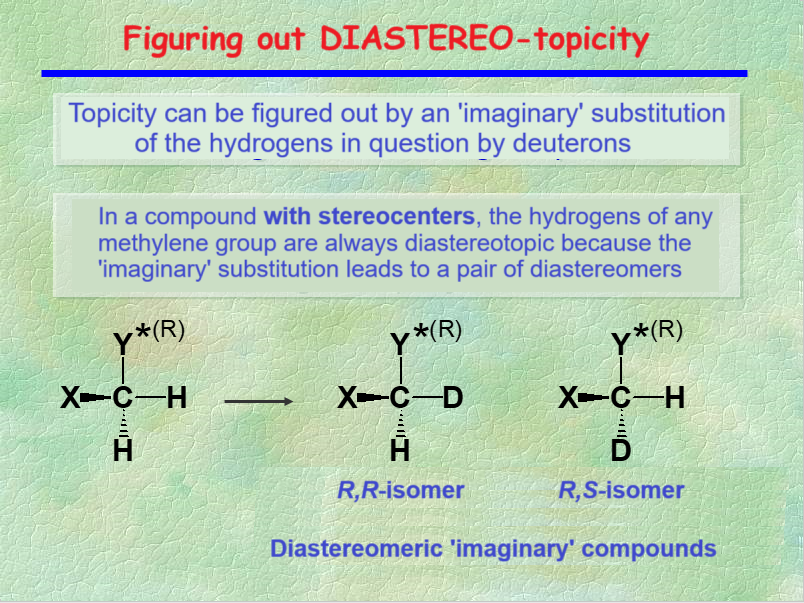
TOPICITY can be guessed by the "trick" of the imaginary isotopic substitution.
Nuclei are HOMOTOPIC when the imaginary isotopic substitution leads to the very same compound.
HOMOTOPIC nuclei are ISOCRONOUS, i.e. they resonate at the very same chemical shift.
The 1H's from a CH2 group of a molecule without any stereocenter are ENANTIOTOPIC, as it is shown by the imaginary isotopic substitution that leads to a enantiomer pair.
ENANTIOTOPIC nuclei are always ISOCRONOUS, i.e. they show up at exactly the very same chemical shift.
The 1H's from a CH2 group of a molecule with at least one stereocenter are DIASTEREOTIOTOPIC, as it is shown by the imaginary isotopic substitution that leads to a diastereomer pair.
DIASTEREOTOPIC nuclei can resonate at different chemical shift, i.e. they cannot be ISOCRONOUS.
In general, they will show up at different chemical shift but accidental coincidence can never be discarded.
To your astonishment, a molecule without stereocenters can bear DIASTEREOTOPIC nuclei. Am I serious?
An excelent ejemple is glycerin HOCH2CH(OH)CH2OH.
Check this out:
The imaginary isotopic substitution of one 1H in any of the CH2 groups gives rise to the creation of a stereocenter at the central carbon thus making the 1H's of each CH2 group be DIASTEREOTOPIC.
Isn't it weird enough?