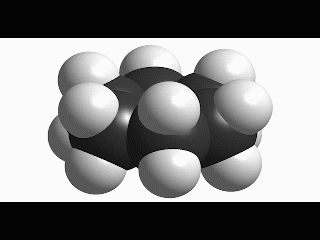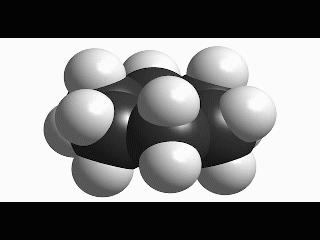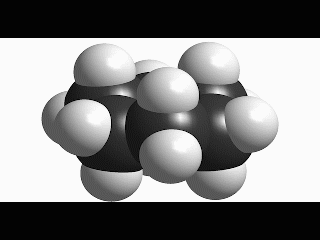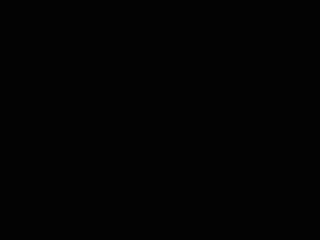
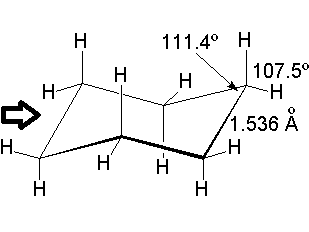
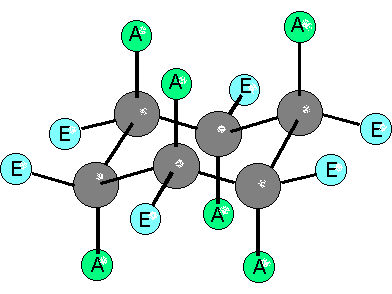
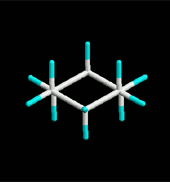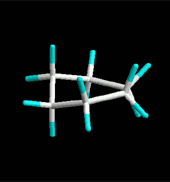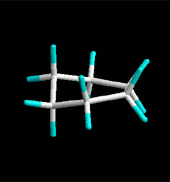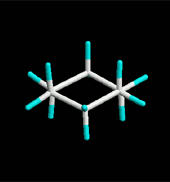
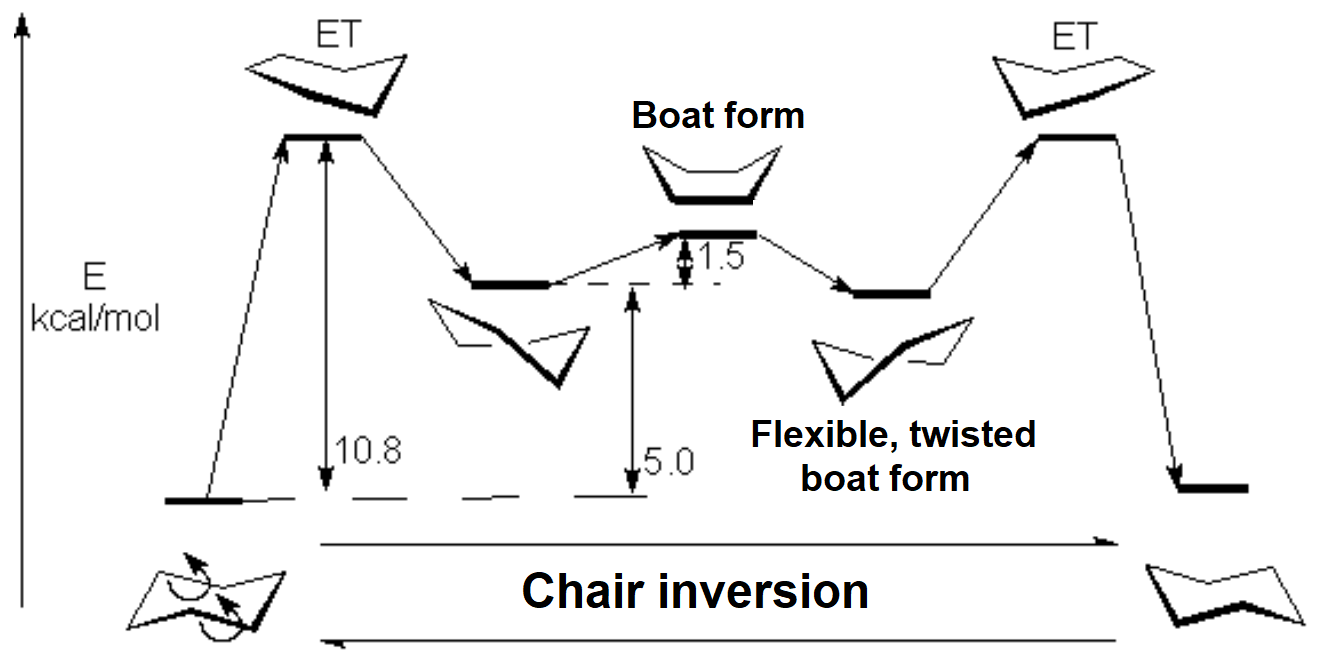
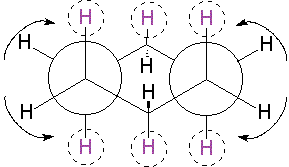
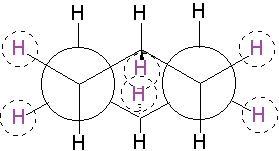
Since cyclohexane is built from single C-C bonds, their rotation is feasible. The combined rotation of various bonds in cyclohexane gives rise to a conformational equilibrium where the chair inverts.
Please note that the chair inversion produces an exchange of positions: all ecuatorial hydrogens move to be axial and viceversa.
The mechanism of combined bond torsion is quite complex and implies the passing through 'boat' and 'twist boat' conformers, with a global inversion barrier of 11 kcal/mol, four time as much as in ethane.
However bigger is the barrier in cyclohexane, the chair inversion happens several thousands times per second.
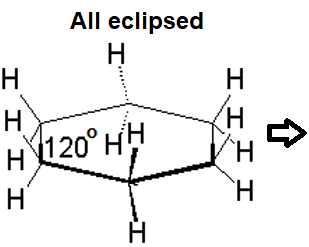 If cyclohexane were planar, every three consecutive carbons would share a 120º angle, very inadequate for a sp3 hybridization. Besides, all hydrogens would be eclipsed.
If cyclohexane were planar, every three consecutive carbons would share a 120º angle, very inadequate for a sp3 hybridization. Besides, all hydrogens would be eclipsed.