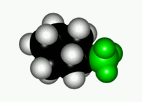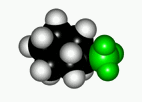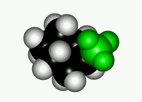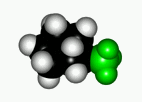
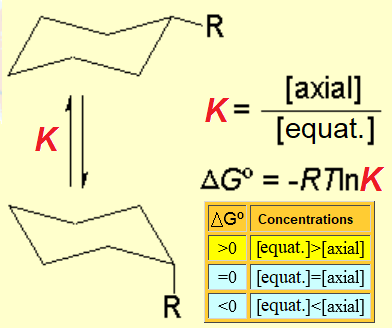
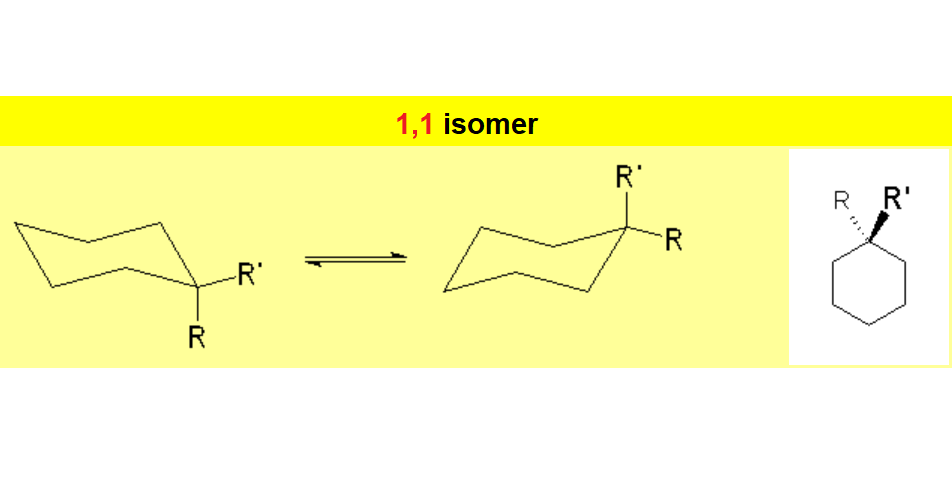
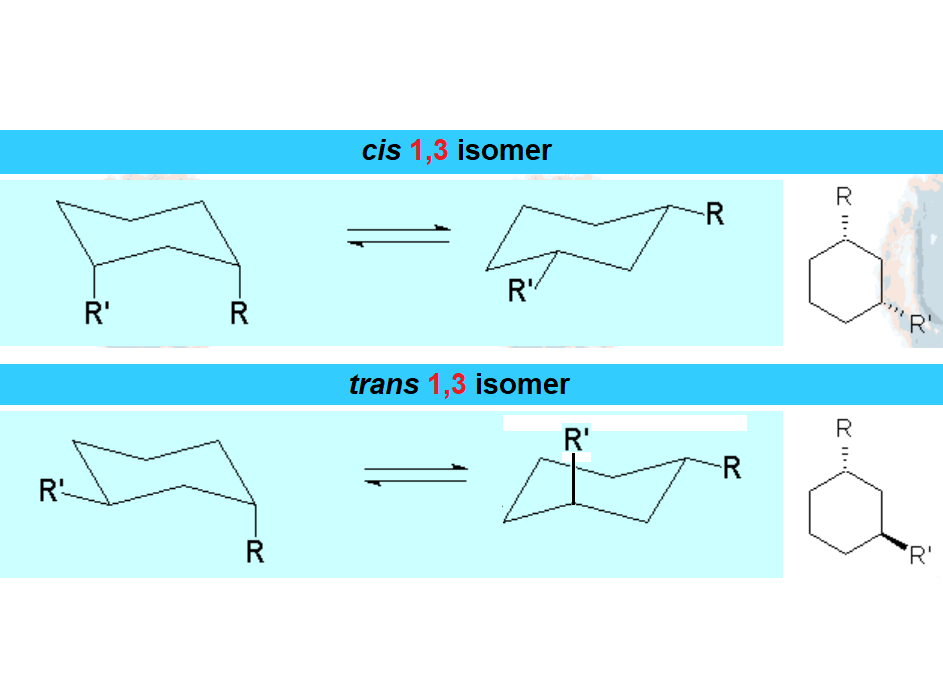
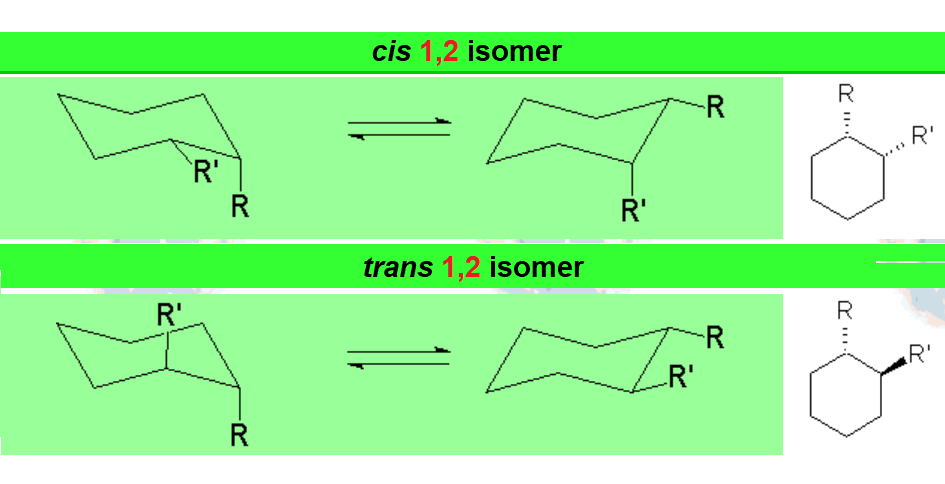
When a cyclohexane ring bears at least one substituent, the two chairs in equilibrium stop having the same energy.
In general, the chair form with the most voluminous substituent(s) in equatorial is the most stable one, as it is shown by the following experimental results.
If we set the chair-to-chair equilibrium as shown, its upwards shift towards the equatorial conformer implies a positive change in free energy, as displayed by the equations:
Are we able to understand why the methyl group has an experimental conformational energy of 1.8 kcal/mol?
Please, recall that the gauche interaction between two methyl groups in butane had an energy cost of 0.9 kcal/mol.
That's why an axial methyl group is less stable than an equatorial one by double 0.9 = 1.8 kcal/mol.
In general, the substituents are less stable in axial arrangement due to their interaction with the methylene groups at relative positions 3 and 5.
Depending on the size of the substituent, its interaction would be more or less severe and its conformational energy bigger or smaller.
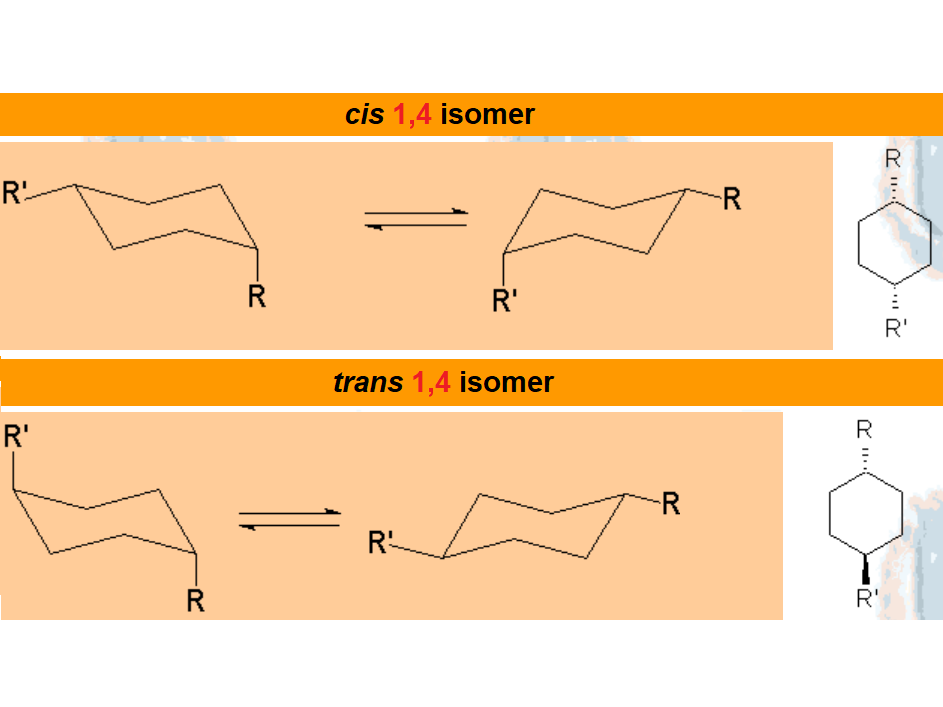
In disubstituted cyclohexanes, the conformational equilibrium will be shifted towards the conformation with less substituents in axial and/or the chair bearing the largest substituent in equatorial.