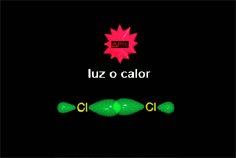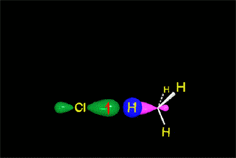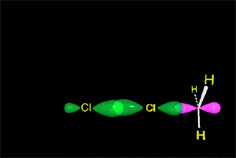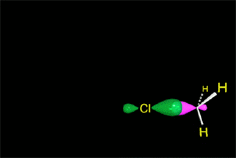
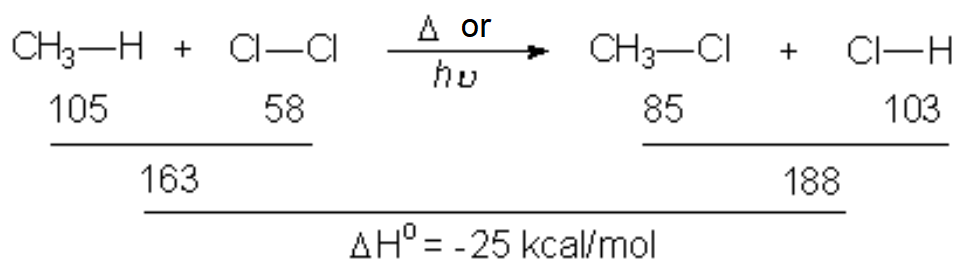
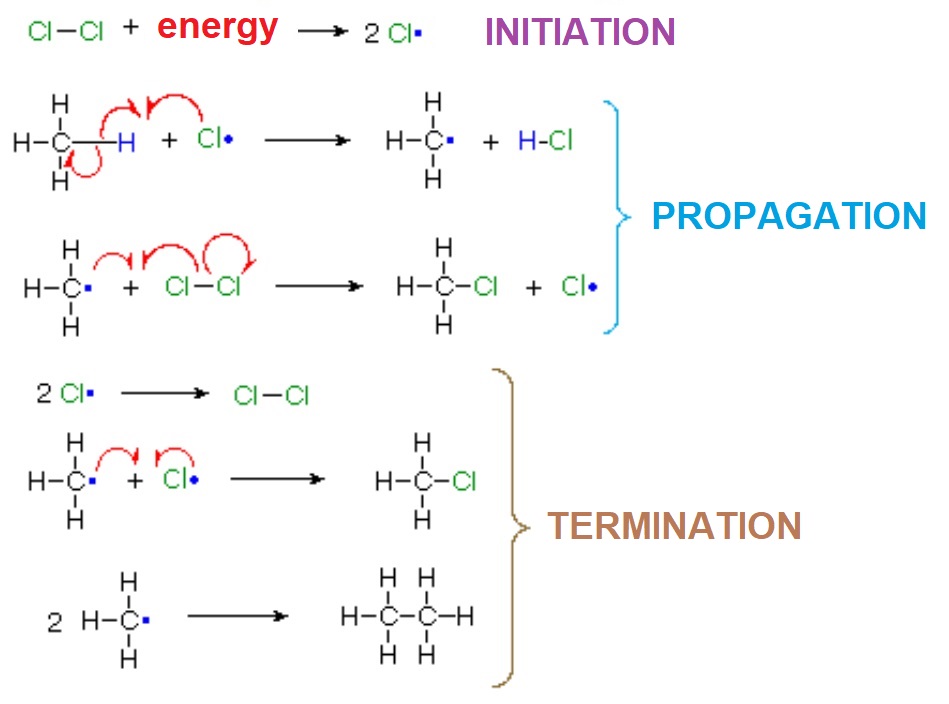
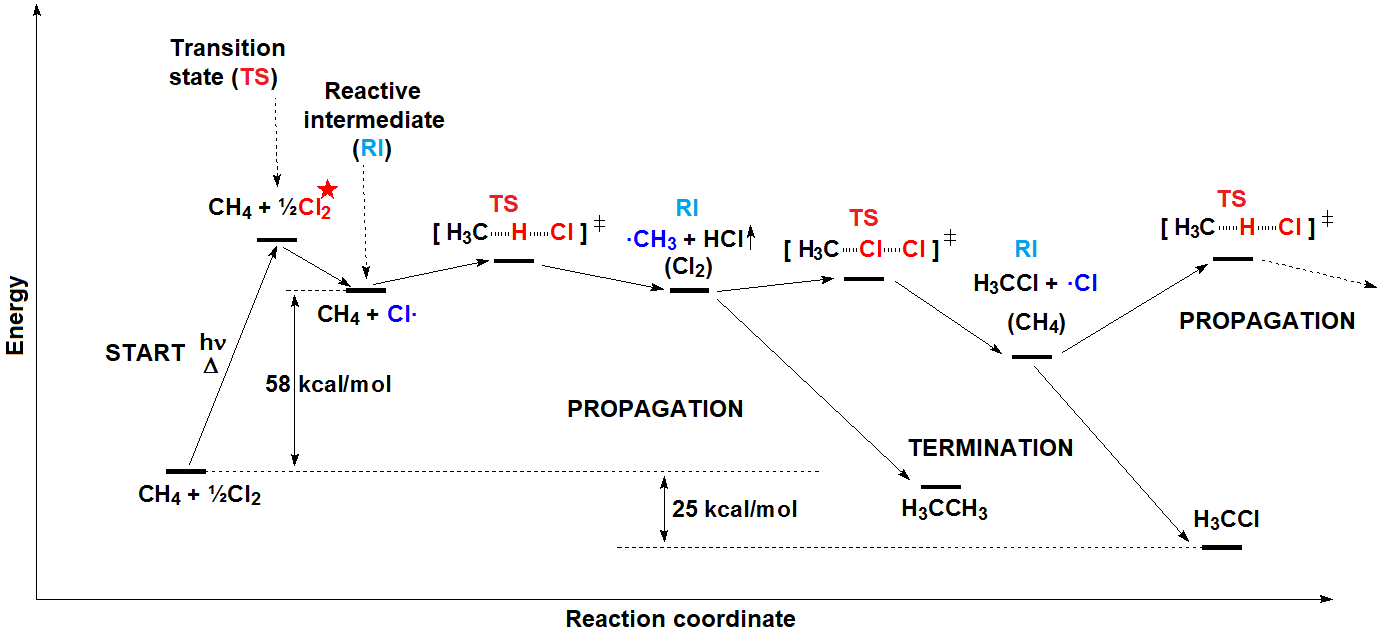
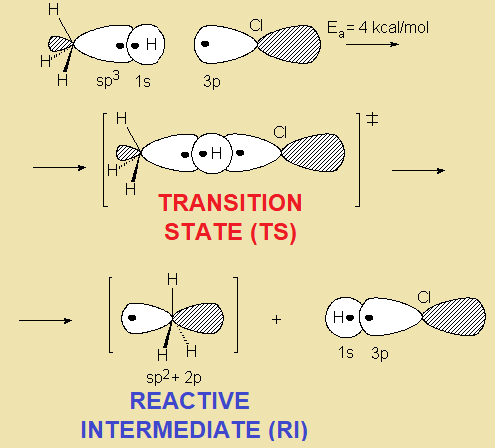
In a reaction sequence, there exist TRANSITION STATES, where bonds are either breaking or forming, and REACTIVE INTERMEDIATES, which are transient species, of relatively high energy but local minima in the general reaction scheme.
As a reaction progresses, the reactants get closer and, if an effective collision occurs, the bond breaking and/or forming takes place.
This is not a favorable process because, at short distances, molecules repel each other.
The potential energy increases until a state of maximum energy is reached, the so-called TRANSITION STATE, in short TS.
The necessary energy for the reagents to reach the TS is the ACTIVATION ENERGY, which is the energy that precludes the exothermic reactions to take place spontaneously.
Once the TS is overcome, the process can directly lead to the final products or, more commonly, to a RACTIVE INTERMEDIATE (in short RI) that, through a new TS, leads to the final products or, in more complex mechanisms, to a new RI, then to a new TS and so on and so forth.
A reaction mechanism is the sequential reckoning of all possible TS's and RI's through which the reaction is expected to take place.
The most important carbon RI's in organic reactions are the carbocations (R3C+), the free radicals (R3C·), the carbenes (R2C:) and the carbanions (R3C-).