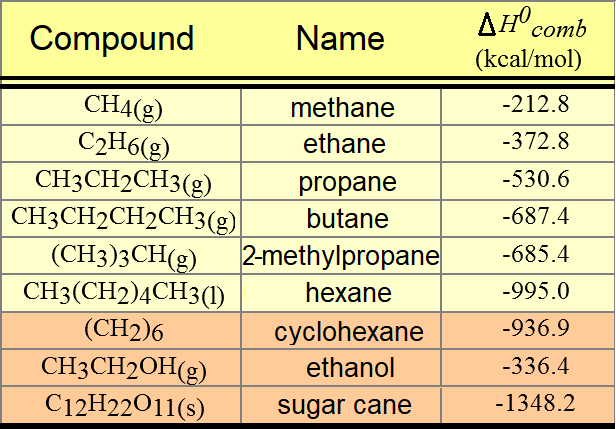
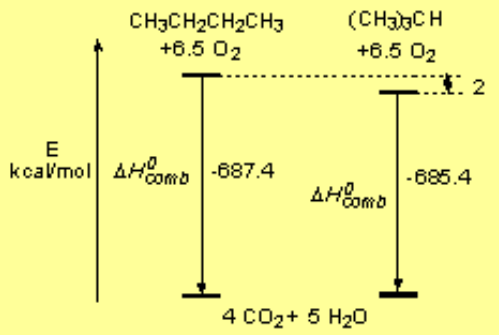
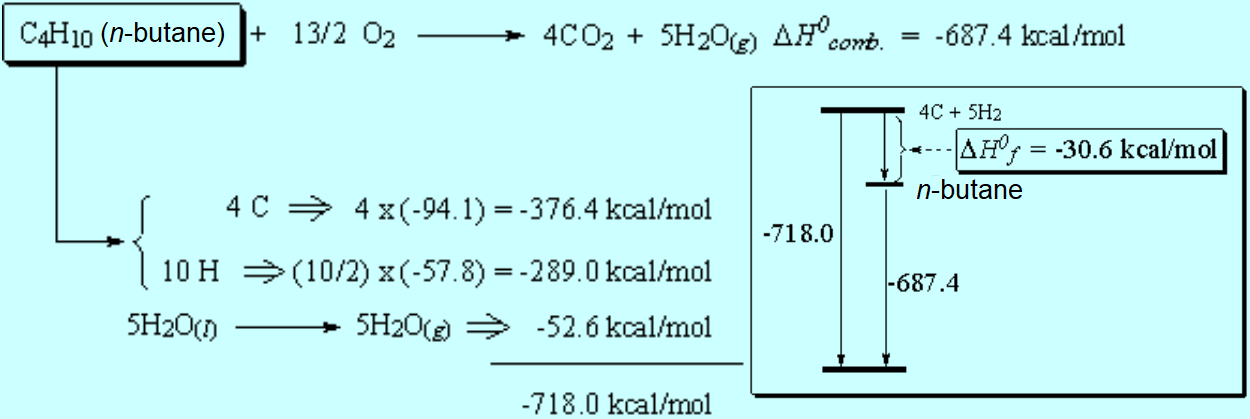
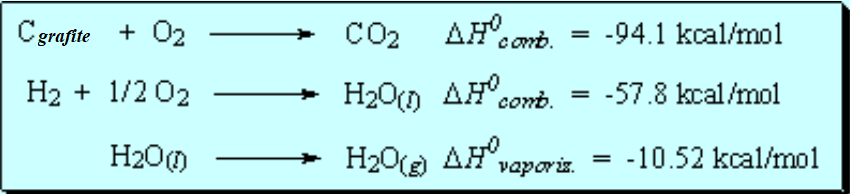The difference between the 'real' heat of combustion and the 'theoretical' one calculated from the elements is the heat of formation.
If the latter ends up as a negative value, it means that the compound is more stable than its isolated elements. This is the normal pattern but not always happens.