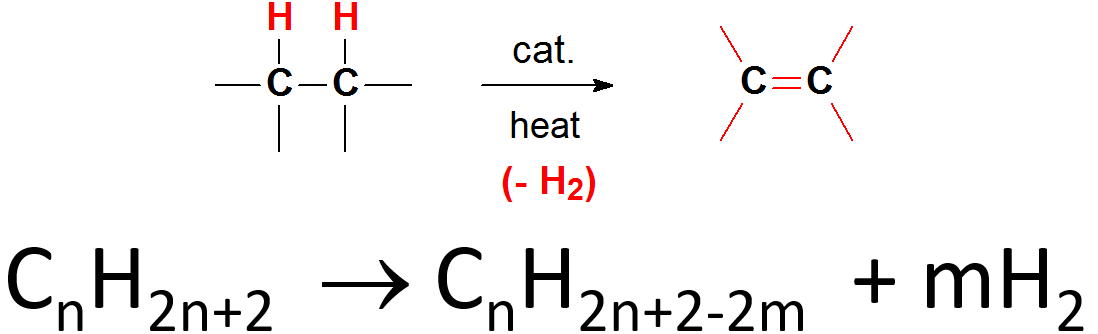Dehydrogenation is one of the most important processes in the chemistry of petroleum because it turns the starting inert alkanes into olefins and aromatic compounds, starting points towards other functional groups.
Oxidation is the counterpart to reduction. Reduction is synonymous to hydrogen gain, the opposite to dehydrogenation.
Therefore, chemically speaking, dehydrogenation and oxidation are also synonyms.
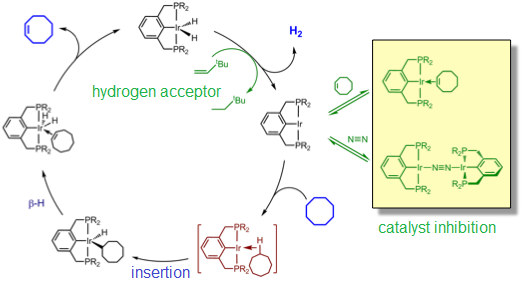
This is the example of the dehydrogenation of cyclooctane, by means of an Iridium catalyst bearing a 'tweezer' shape. The catalyst molecule holds the metal as it were a pair of tweezers.
This is an excellent example of what is called a 'catalytic cycle'. This is a kind of mechanism that describes how the reacting compound (cyclooctane) binds to the catalyst and the transformations it suffers. There is too a 'sacrificial' compound (tert-butylene) that accepts hydrogen from the dehydrogenation.
The cycle also shows how the catalyst 'gets old' by the binding of species that make it inactive.