RELATIVE STABILITY OF CYCLOALKANES
The relative stability of cycloalkanes depends on their size and hence, on the tension they have within their rings.
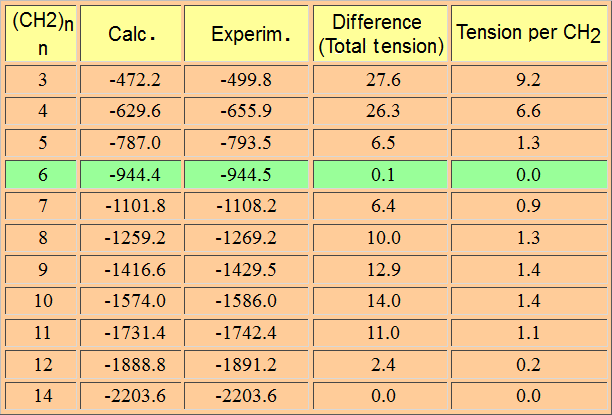
By observing the table of heats of combustion one can deduce that every added CH2 group makes the value more negative by 157.4 kcal/mol.
We can thus calculate the expected combustion heat (157.4 x n) of any cycloalkane (CH2)n.
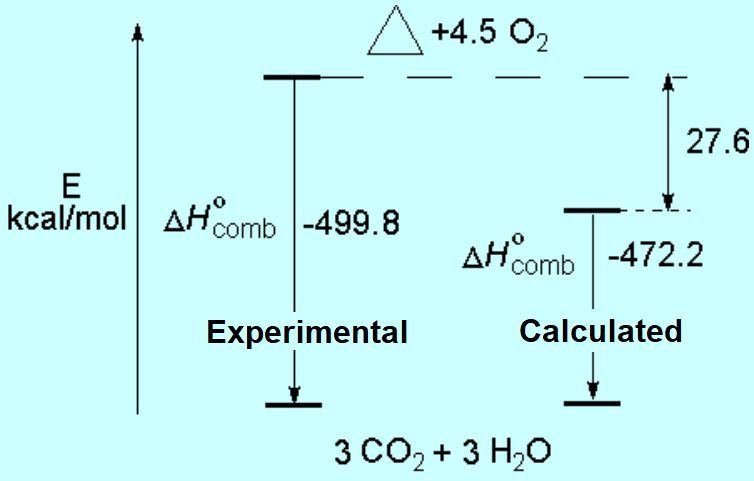
In the case of cyclopropane, the combustion is more exothermic than expected.
That means that the CH2 groups of cyclopropane are not 'normal' because they result higher in energy -less stable- than anticipated.
The difference in expected and real energy is the consequence of the tension present in a three-membered ring.
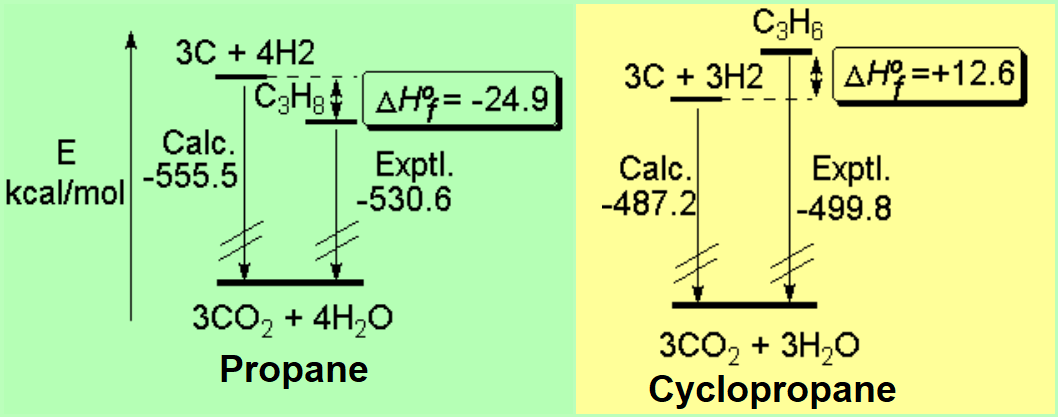
We may use the calculation of the heats of formation to compare the relative stability of cyclopropane and propane.
Propane means a more stable situation -by ca. 25 kcal/mol- than its separated elements.
In turn, cyclopropane is a less stable stage -by ca. 13 kcal/mol- than its separated elements.
That doesn't preclude cyclopropane from existing but the heat of formation strongly suggests the ring tension.
Cyclopropane would be more reactive because it would tend to be freed from the ring tension by ring aperture.
Small cycloalkanes are less stable than expected as compared to 'normal' alkanes. What is the origin of that 'abnormality'?
In an acyclic alkane the frontal overlapping of the sp3 orbital should be maximum, giving rise to a C-C bond of high strength (deltaHºC-C = 90 kcal/mol).
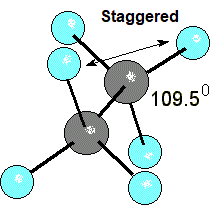
On the other hand, the hydrogens can easily adopt an alternated conformation where their steric and orbital interactions are minimized.
 The internal angle in cyclopropane is 60º, much smaller than the natural angle of tetrahedral sp3 orbitals (109.5º).
The internal angle in cyclopropane is 60º, much smaller than the natural angle of tetrahedral sp3 orbitals (109.5º).
Therefore, the orbital overlapping in cyclopropane cannot be truly frontal and is less perfect.
The experimental lower strength of the C-C bonds in cyclopropane (deltaHºC-C = 65 kcal/mol) is proof of that.
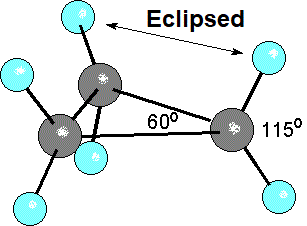 In addition to the distortion in orbital overlapping due to the small size of the ring, the hydrogens on neighboring carbons are inevitably eclipsed.
In addition to the distortion in orbital overlapping due to the small size of the ring, the hydrogens on neighboring carbons are inevitably eclipsed.
That means an added unstability.
The mix of the two factors -ring tension and hydrogen eclipsing- explain the difference between the calculated heat of combustion -as if cyclopropane were a 'normal' alkane- and the experimental one.
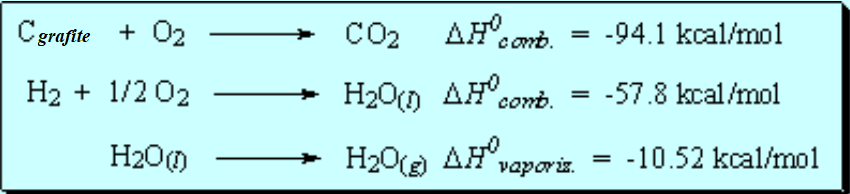
 The internal angle in cyclopropane is 60º, much smaller than the natural angle of tetrahedral sp3 orbitals (109.5º).
The internal angle in cyclopropane is 60º, much smaller than the natural angle of tetrahedral sp3 orbitals (109.5º). In addition to the distortion in orbital overlapping due to the small size of the ring, the hydrogens on neighboring carbons are inevitably eclipsed.
In addition to the distortion in orbital overlapping due to the small size of the ring, the hydrogens on neighboring carbons are inevitably eclipsed.