The word PETROLEUM comes from the greek.
It means 'oil of rock'.
It is a dark colored, pungent substance, lighter than water, of a very complex and variable composition, mainly constituted by hydrocarbons, that can be found in nature in not-very-deep underground reservoirs within the earth crust.
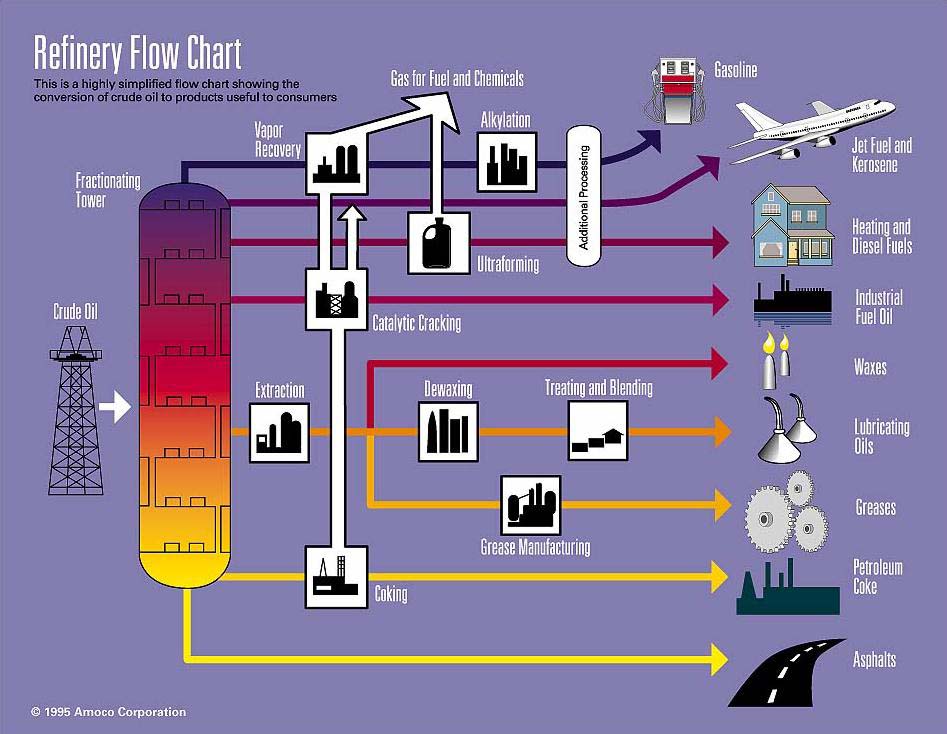 Squeme of a petroleum refinery
Squeme of a petroleum refinery
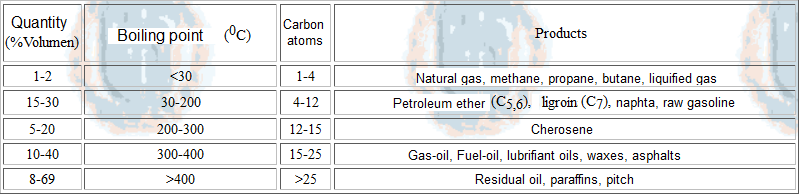
Fractioned distillation of petroleum renders a vast number of different products of crucial industrial importance like gasoline, kerosene, tars, solvents, and a long etc.
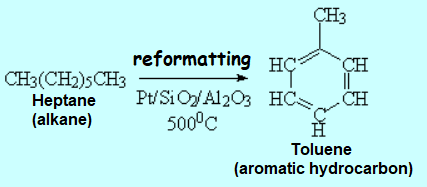
Not a single drop is wasted from petroleum. The heaviest and lightest residues are submitted to different processes, i.e. CRACKING and REFORMATTING, respectively.
By means of CRACKING, the large compounds present in the heaviest residues are broken by the action of high temperatures and special catalysts (a well kept secrect of the companies) among which one can find ZEOLITES.
By REFORMATTING of the lightest residues containing relatively small molecules, more complex molecules can be obtained. The use of special (and secret) catalysts is also essential to this process.
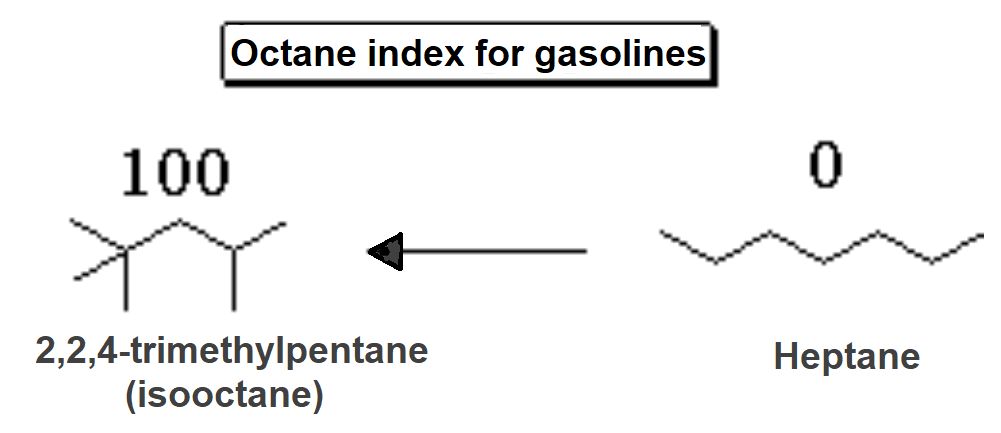 The hydrocarbon with the highest 'octane index' is isooctane. In turn, heptane is the one with the lowest. The 'octane index' indicates the capacity of gasoline self-ignition under pressure. The higher the octane index, the more difficult the self-ignition which is the condition required by the modern combustion engines of vehicles.
The hydrocarbon with the highest 'octane index' is isooctane. In turn, heptane is the one with the lowest. The 'octane index' indicates the capacity of gasoline self-ignition under pressure. The higher the octane index, the more difficult the self-ignition which is the condition required by the modern combustion engines of vehicles.
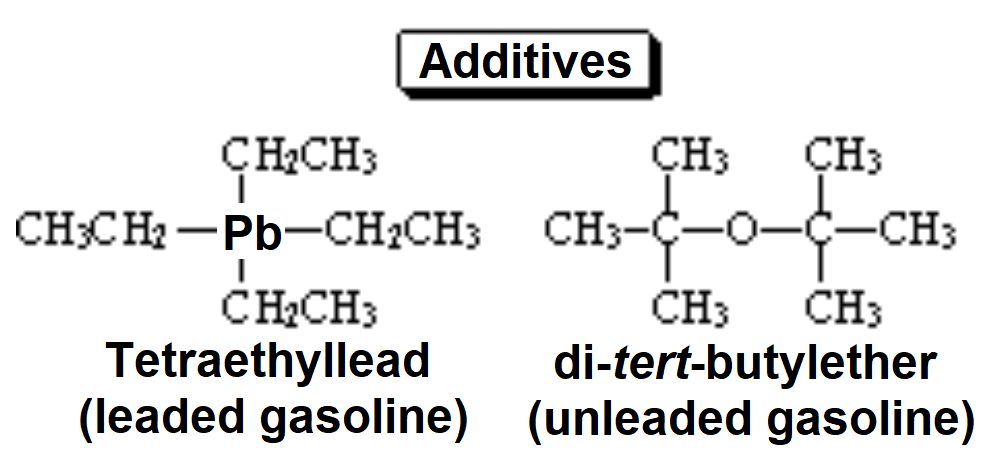 Gasoline would be outrageous expensive if it were constituted only by isooctane in order to get high 'octane index'. The latter can be achieved by mixing different alkanes and the use of additives that avoid the early self-ignition of gasolines in the explosion cycle of an engine. One of the most common additives in the past was tetraethyllead. Since long ago this additive has been banned because of its high toxicity ('unleaded gasolines'). The search for other less harmful additives has been frenetic leading to the use of di-tertbutyl ether.
Gasoline would be outrageous expensive if it were constituted only by isooctane in order to get high 'octane index'. The latter can be achieved by mixing different alkanes and the use of additives that avoid the early self-ignition of gasolines in the explosion cycle of an engine. One of the most common additives in the past was tetraethyllead. Since long ago this additive has been banned because of its high toxicity ('unleaded gasolines'). The search for other less harmful additives has been frenetic leading to the use of di-tertbutyl ether.
 Squeme of a petroleum refinery
Squeme of a petroleum refinery
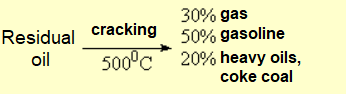

 The hydrocarbon with the highest 'octane index' is isooctane. In turn, heptane is the one with the lowest. The 'octane index' indicates the capacity of gasoline self-ignition under pressure. The higher the octane index, the more difficult the self-ignition which is the condition required by the modern combustion engines of vehicles.
The hydrocarbon with the highest 'octane index' is isooctane. In turn, heptane is the one with the lowest. The 'octane index' indicates the capacity of gasoline self-ignition under pressure. The higher the octane index, the more difficult the self-ignition which is the condition required by the modern combustion engines of vehicles. Gasoline would be outrageous expensive if it were constituted only by isooctane in order to get high 'octane index'. The latter can be achieved by mixing different alkanes and the use of additives that avoid the early self-ignition of gasolines in the explosion cycle of an engine. One of the most common additives in the past was tetraethyllead. Since long ago this additive has been banned because of its high toxicity ('unleaded gasolines'). The search for other less harmful additives has been frenetic leading to the use of di-tertbutyl ether.
Gasoline would be outrageous expensive if it were constituted only by isooctane in order to get high 'octane index'. The latter can be achieved by mixing different alkanes and the use of additives that avoid the early self-ignition of gasolines in the explosion cycle of an engine. One of the most common additives in the past was tetraethyllead. Since long ago this additive has been banned because of its high toxicity ('unleaded gasolines'). The search for other less harmful additives has been frenetic leading to the use of di-tertbutyl ether.