UNIMOLECULAR ELIMINATION (E1)
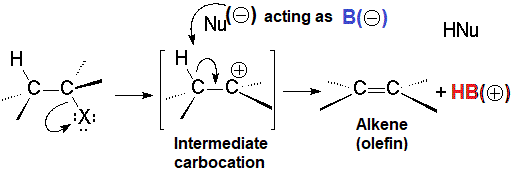
The carbocation produced when the leaving group departs can evolve in a different way to the SN1 reaction:
Elimination of a proton at its vicinal position.
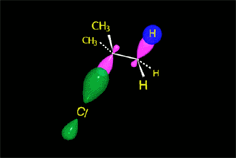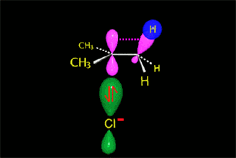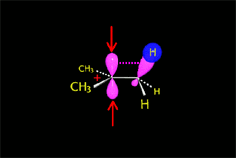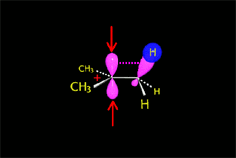 Carbocation (SN1)
Carbocation (SN1)
 Carbocation (E1)
Carbocation (E1)
The nucleophile, ACTING AS A BASE, can extract a proton from the vicinal carbon to the carbocation, leading to an olefin.
In the case of the methanolysis of tert-butyl chloride, it is the methanol that acts as a base, taking the proton and producing isobutylene.
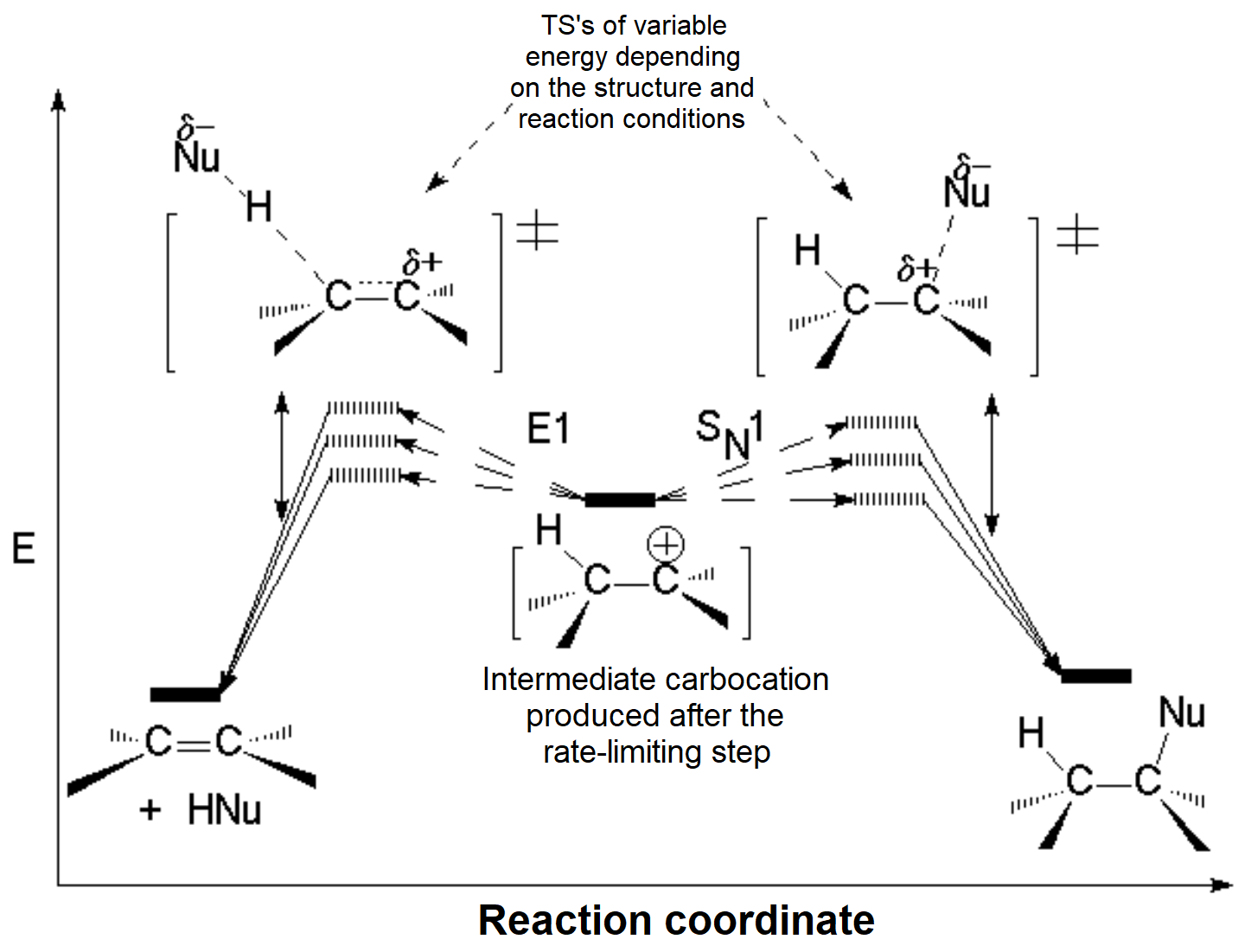
As mentioned above, the carboction can evolve in two different pathways.
The energy diagrams are as follows:
The substitution-to-elimination ratio does not depend on the leaving group because the carbocation is a common intermediate in both pathways but it does on the basicity/nucleophilicity relatonship of the attacking base/nucleophile.
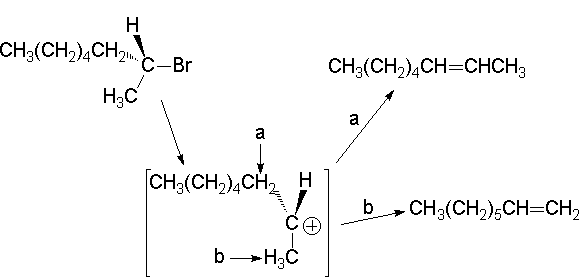
Any vicinal hydrogen to the carbon bearing the leaving group can participate in the E1 reaction. Consequently, the carbocation generated from 2-bromooctane can lead to either 1- or 2-octene:
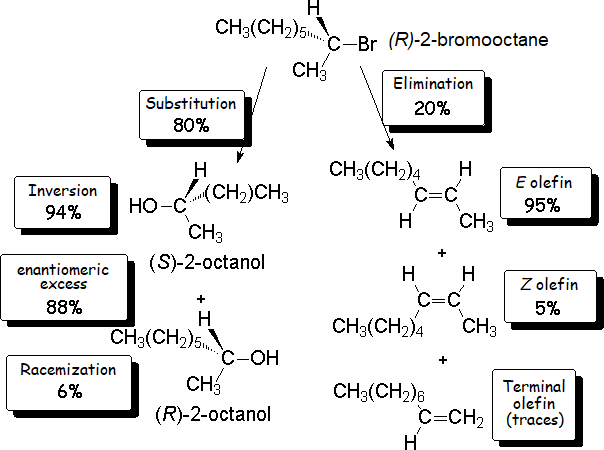
We have now explained why our 'experiment' led to the formation of 20% olefins.
We only have to understand why only traces of 1-octene are produced and why trans-2-octene is the major olefin.
Those facts will be explained in the alkenes' chapter.
 Carbocation (SN1)
Carbocation (SN1)
 Carbocation (E1)
Carbocation (E1)