BIMOLECULAR ELIMINATION (E2)
There still exists another elimination mechanism:
The bimolecular eleimination (E2).
Look at it in motion!!!
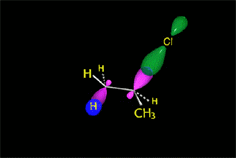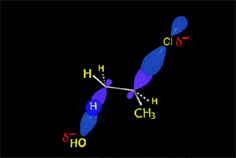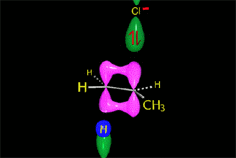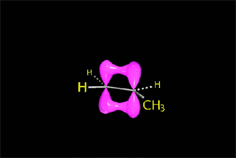
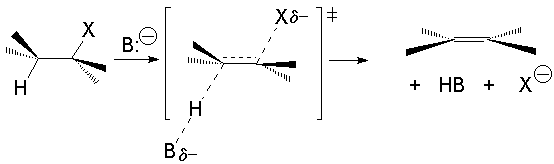
The bimolcular elimination (E2) takes place without reactive intermediathes through a single TS, where the base detaches the proton at the same time the leaving group departs.
The two involved carbons change hybridization from sp3 to sp2.
E2 kinetics depends on both the halide and the nucleophile and it is thus second order: The E2 reaction is bimolecular.
The E2 reaction proceeds with a fixed stereochemistry: the leaving group and the hydrogen on the vicinal position depart in ANTIPERIPLANAR arrangement.
The following experimental data is proof of the E2 mechanism and of its tight stereochemical requirements:
The meso isomer of 2,3-dibromobutane exclusively renders the E olefin by HBr elimination, whereas any of the other two diastereomers (R,R) or (S,S) or the racemic mixture lead to the Z olefin
Would this STEREOSPECIFICITY be possible if the mechanism weren't E2?
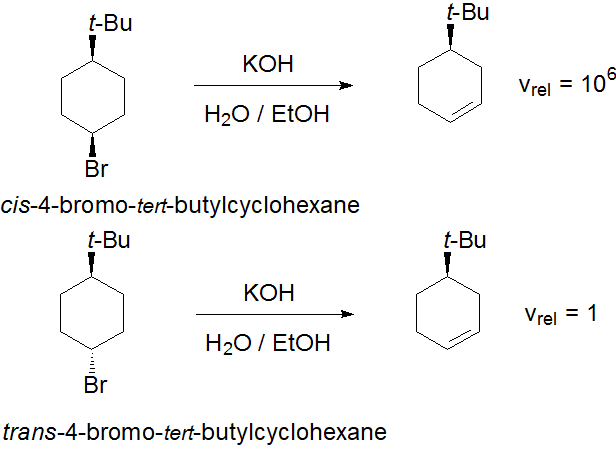
On the other hand, is there any way to explain the different elimination rate displayed by the cis and trans isomers of 4-bromo-tert-butylcyclohexane but by an E2 mechanism?
In order to do that, consider which conformer is the most populated in each isomer's equilibrium...
Which of the two bears the H and Br in ANTIPERIPLANAR arrangement?