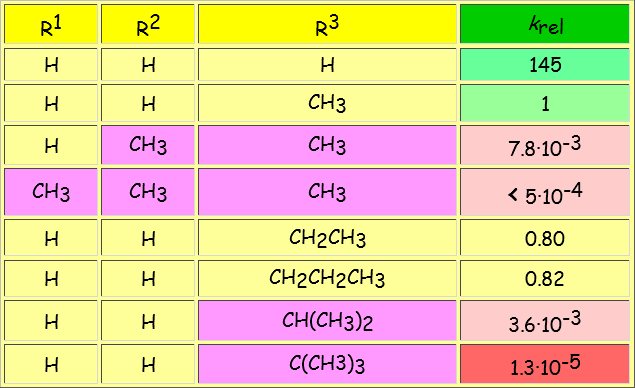
The SN2 reaction is very sensitive to steric hindrance because, in the TS, the reacting carbon is a trigonal bipyramid where there are five groups in close proximity.
The volume of the R groups critically determines the energy of the TS and hence the reaction rate.