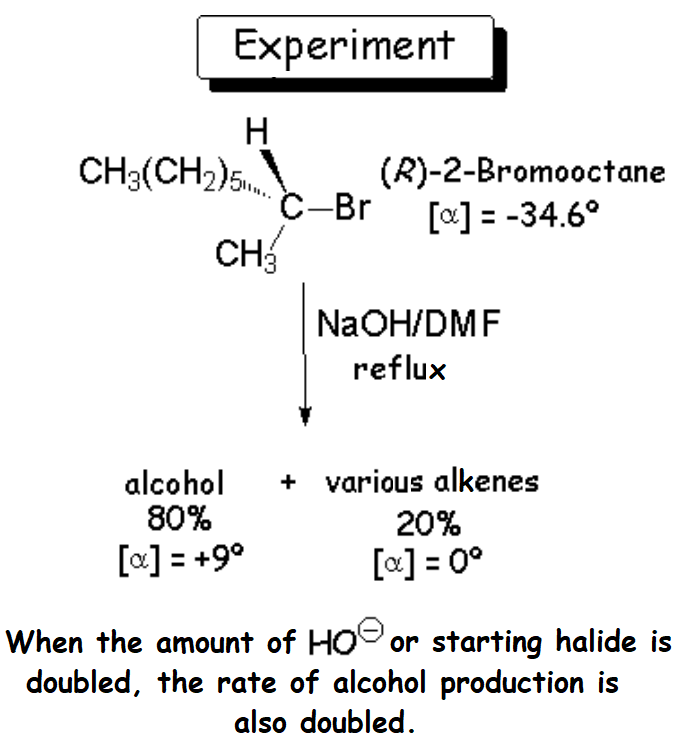
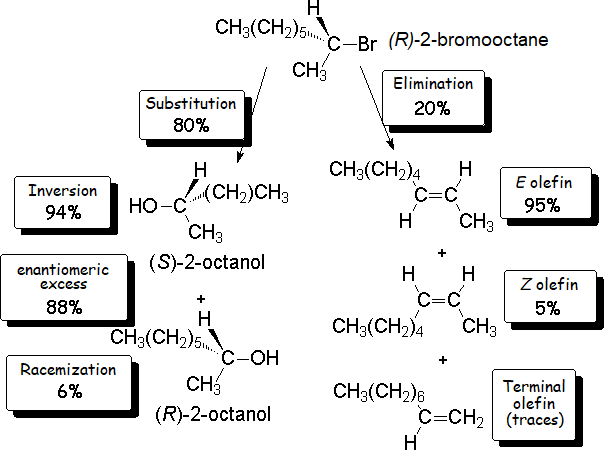
The thorough analysis of the reaction mixture tells us that the result is the major production (80%) of an optically active alcohol,
together with a series of optically inactive alkenes.
It is reasonable to think that the nucleophile (HO-) has attacked the haloalkane by the electron-deficient carbon of 2-bromooctane, that bonded to the bromine.
Consequently, the alcohol should be 2-octanol.
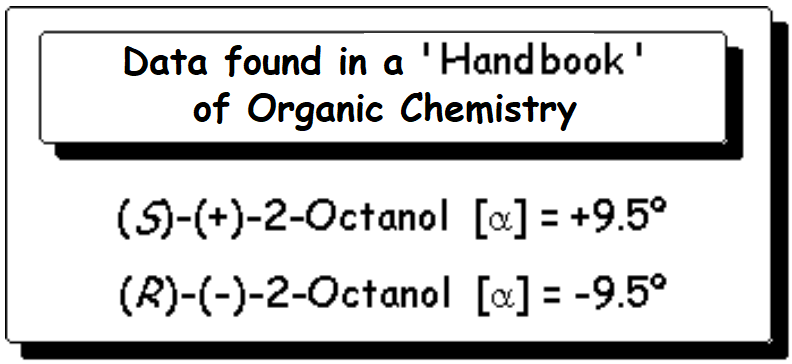
Let's check a chemical catalog, where we can find the following data: