FORMATION OF ORGANOMETALLIC
REAGENTS
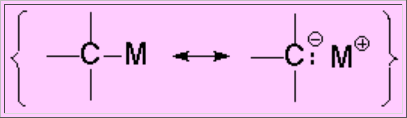
A carbon bonded to a metal, of much lower electronegativity, becomes an electron rich center and hence a strong nucleophile.
The C-Metal bond is very polar.
The organometallic compounds are generally prepared by the treatment of an halogenated derivative with a metal.
THe most common metals are lithium and magnesium. The magnesium organometallic compound are called Grignard reagents.
Víctor Grignard (1871-1935): French Chemist. In 1910 discovered the organomagnesium reagents that were called after him. He won the Noble Prize in 1912, shared with Sabatier. He authored a chemistry treatise of 20 volumes.
Organometallics have to be used right after their preparation bacause they are so reactive that get easily and violently destroyed by oxygen and moisture.
Their preparation and use must be carried out under inert atmosphere (N2 or Ar).
The organometallic reagents are very good carbon nucleophiles and allow us the building of new C-C bonds, very important for the development of new compounds in Organic Chemistry.
Here you are some examples: