UNIMOLECULAR NUCLEOPHILIC SUBSTITUTION (Sn1)
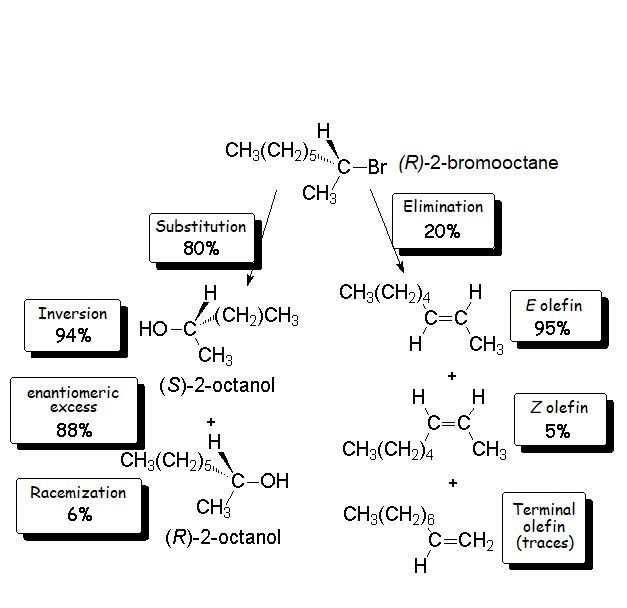
We already know the SN2 mechanism that accounts for (S)-2-octanol as the major 'inverted' product of our experiment.
But, how can we explain the 6% formation of (R)-2-octanol?
We have to draw on an alternative mechanism: unimolecular nucleophilic substitution (SN1).
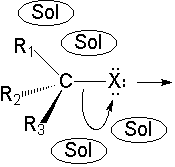
C-Br bonds are highly polarized towards the more electronegative halogen atom.
The presence of the polar solvent may prompt the spontaneous heterolytic cleavage of the C-Br bond, the resulting ions being more or less separated (solvated) by the solvent.
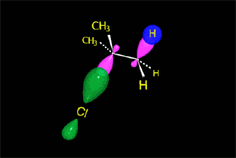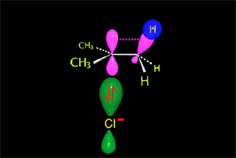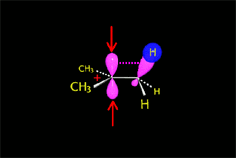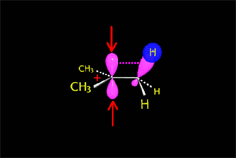
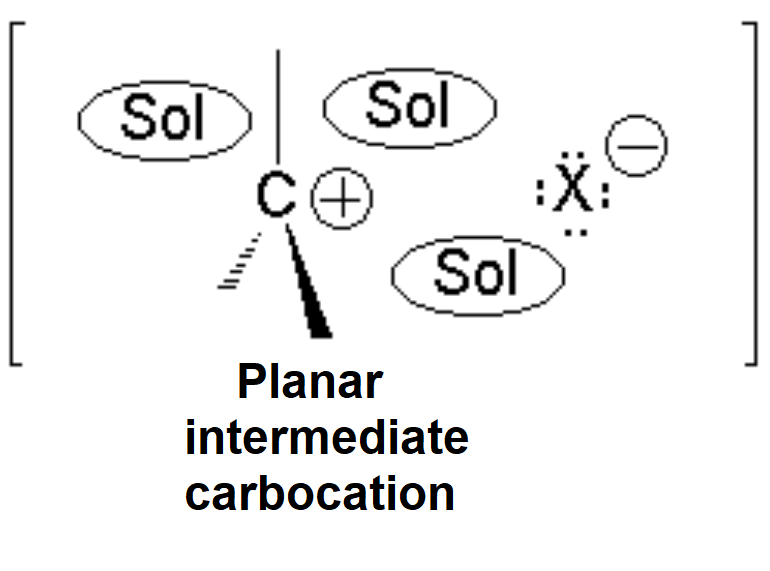
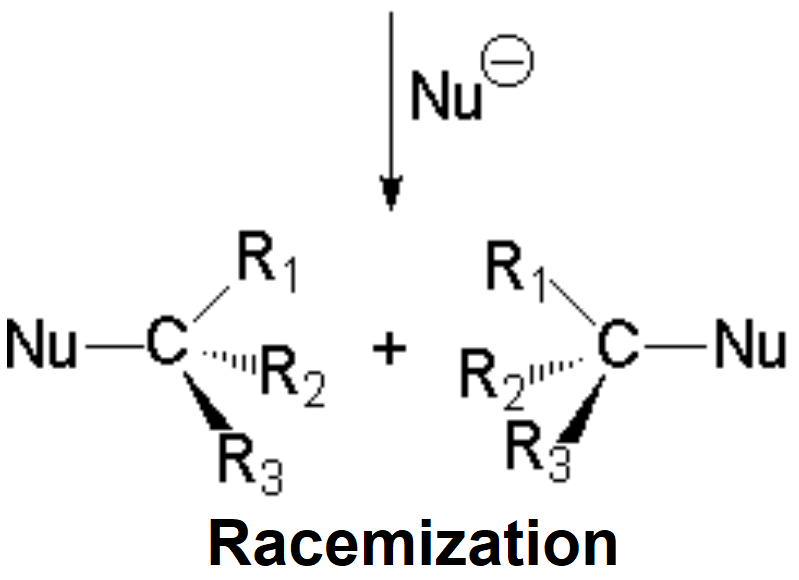
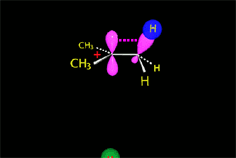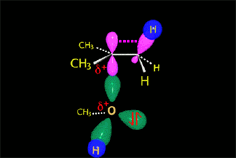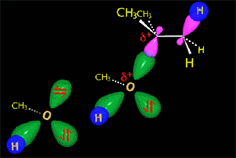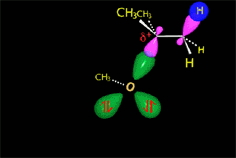
The so-formed carbocation is planar, the charged carbon being sp2 hybridized.
The empty p orbital, orthogonal to the plane, can be equally attacked by any of its two lobules.
The attack of the nucleophile by either of the two sides gives rise to the two possible enantiomers.
Since the probability is the same, this mode of attack renders the two enantiomers in the same ratio, i.e. it produces a recemic mixture.
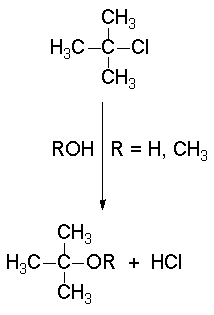
The SN1 reactions - a good example is the solvolysis of tert-butyl chloride - display a 'first order' kinetics.
Rate = k[(CH3)3CCl] mol/L·s
This contrasts with the 'second order' kinetics of the SN2 couterpart mechanism.
This 'first order' kinetics, only depending on the haloderivative, means that, in the SN1 mechanism, the haloderivative is the sole player in the rate-limiting step.
Look at the differences in the two cartoons:
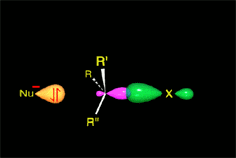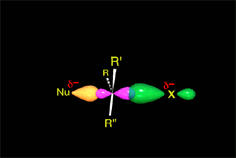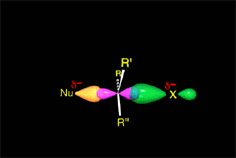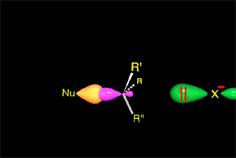 SN2
SN2
 SN1 (carbocation)
SN1 (carbocation)
The carbocation is attacked by the nucleophile.
In the solvolysis of tert-butyl chloride the solvents also acts as nucleophile that collapses with the carbocation.
The respective energy profiles of the SN1 and SN2 processes are as follows:
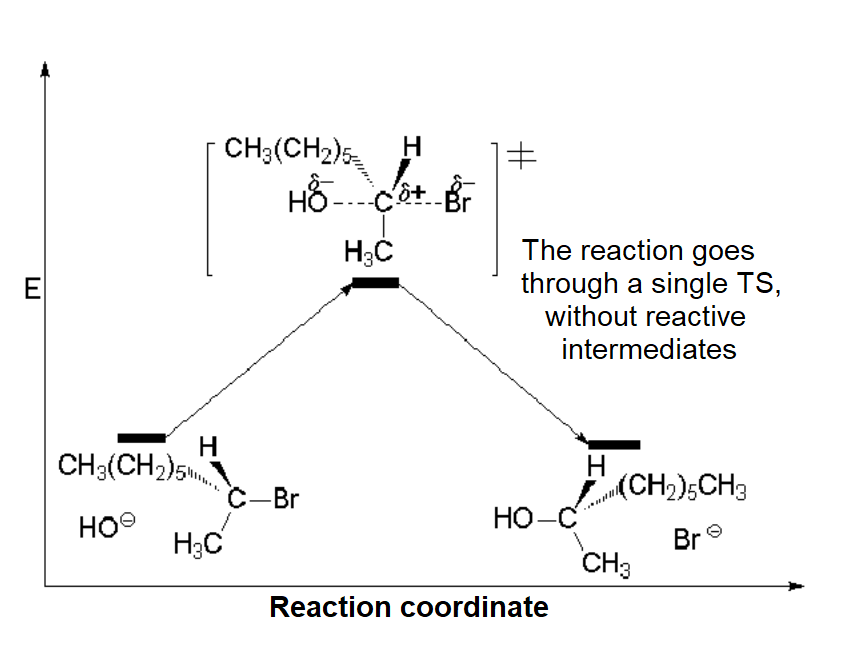 SN2
SN2
 SN1 (carbocation)
SN1 (carbocation)
The SN1 reaction lacks stereospecificity because it takes place through the formation of a planar carbocation that can be attacked equally by either of its two sides.
In the substitution reaction the SN1 and SN2 processes compete between them.
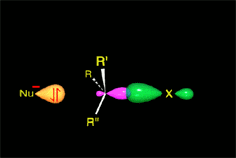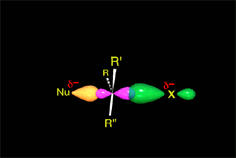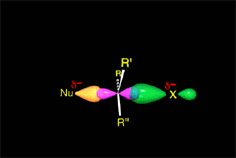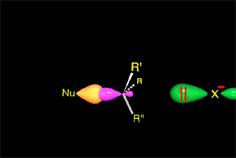 SN2
SN2
 SN1 (carbocation)
SN1 (carbocation)
 SN2
SN2
 SN1 (carbocation)
SN1 (carbocation)