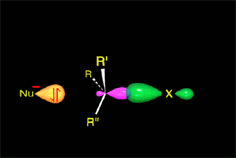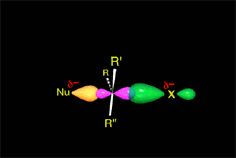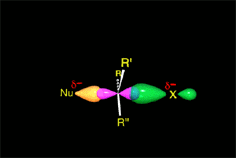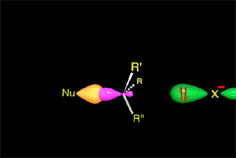Sn2: KINETICS, MECHANISM AND STEREOCHEMISTRY
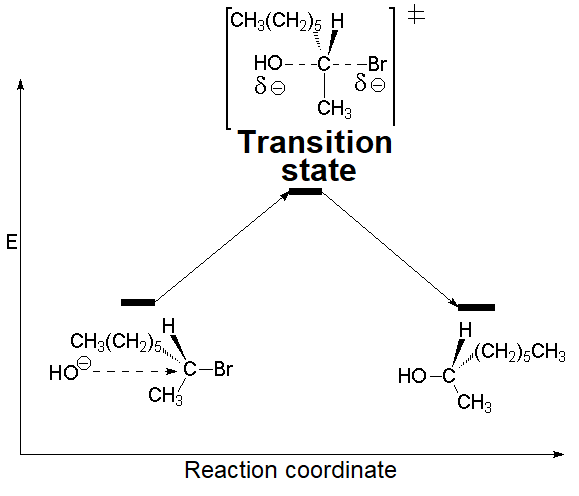
Reaction kinetics of the nucleophilic substitution of (R)-2-bromooctane with hydroxide is second order.
V = k[ C8H17Br ][ HO- ] mol/L·s
A reaction following such 'second order' kinetics is called bimolecular because both substrate and nucleophile intervene in the rate-limiting 'transition state' (TS).
The nucleophile attack and the leaving group expulsion must be synchronized.
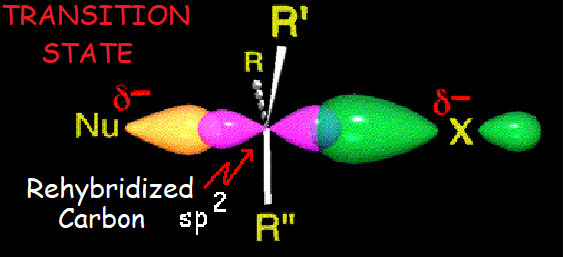
This synchronization can be understood by a TS in which the carbon suffering the attack is momentarily pentacoordinated, with a sp2 hybridization.
The attack begins...
The nucleophile approximates by the opposite face to where the leaving group is.
An occupied orbital of the nucleophile starts interacting with the small lobule of the sp3 carbon orbital still sustaining the leaving group.
Only this kind of approximation is able to render an effective reaction.
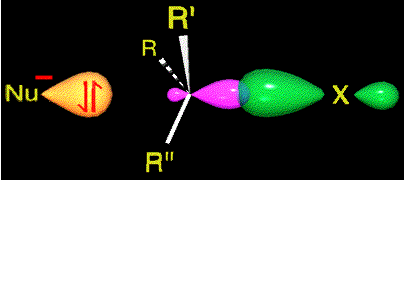
The activation energy is overcome and the system arrives to the TS.
The attacked carbon is transitorily pentacoordinated, in sp2 hybridization. Carbon's p orbital is doubly bonded to the nucleophile and the leaving group.
The R groups are on a plane.
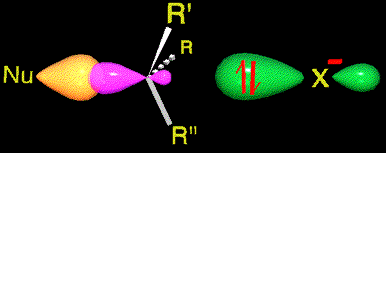
The TS tension, which amounts for the activation energy, is liberated by the expulsion of the leaving group.
The carbon gradually recovers its initial sp3 hybridization back.
The configuration of the attacked carbon ends up inverted.
The easiness to carry out a SN2 reaction depends on:
1. The nature of the leaving group
2. The reactivity of the nucleophile
3. The steric factors exerted by the R groups.