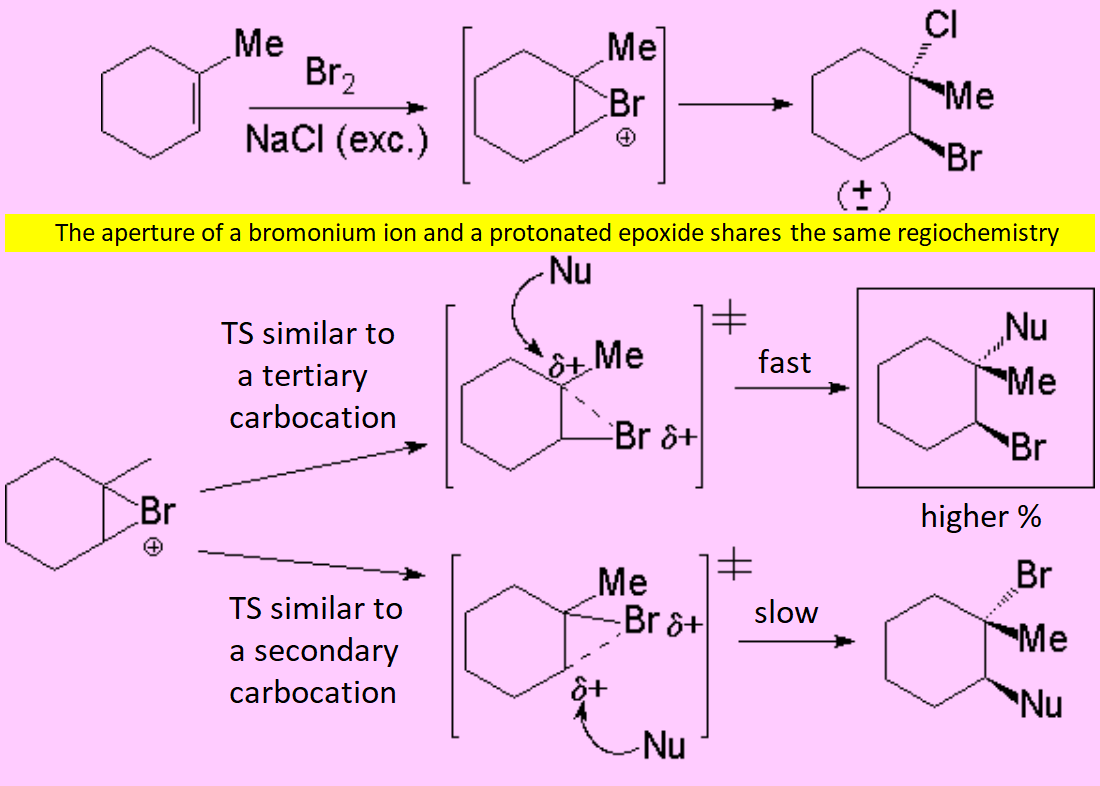
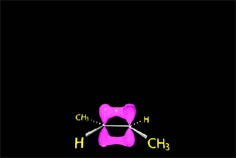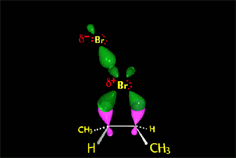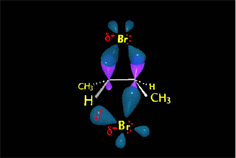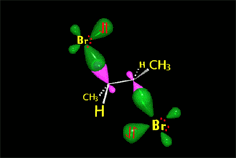
The "pi" cloud of the double C=C bond can induce the heterolytic cleavage of the bromine molecule, giving rise to an intermediate with the structure of heterocyclopropane
(halonium ion).
The cyclic intermediate is opened by the halide counterion, leading to a vicinal dihalo derivative of anti stereochemistry.
 The reaction is only of practical use for Cl2 y Br2.
The reaction is only of practical use for Cl2 y Br2.
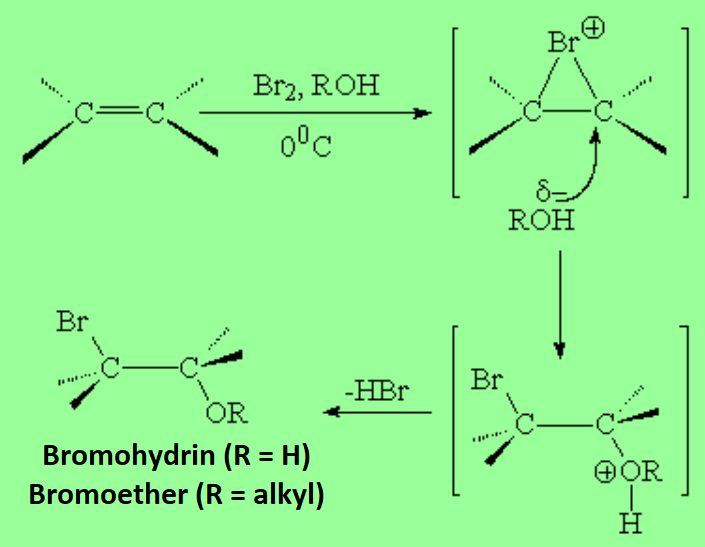
If the nucleophile is water or alcohols, one gets halohydrins or haloethers, respectively.
This is an excellent way of functionalizing two carbons in one shot.
 The reaction is only of practical use for Cl2 y Br2.
The reaction is only of practical use for Cl2 y Br2.